Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Genomics, Other “Omics” Technologies, Personalized Medicine, and Additional Biotechnology Related Techniques
Genomics, “Omics” Technologies, Personalized Medicine
Genomics, Other “Omics”
Technologies,Personalized Medicine, and Additional Biotechnology-Related
Techniques
INTRODUCTION
Pharmaceutical biotechnology and the products result-ing for biotechnologies
continue to grow at an exponential rate. Note that 2005 was a record setting
year with the U.S. Food and Drug Administration (FDA) approval of 21
biopharmaceutical products. There were 15 FDA approvals in 2004, 20 in 2003,
and 13 in 2002 since the last edition of this textbook (Rader 2006; Staff,
2006). Early 2006 saw approvals of several unique new biopharmaceutical
entities including a recombinant vaccine widely hailed as the first vaccine for
the prevention of an oncogenic virus-associated cancer. However, until
recently, the techniques made available by advances in molecular biology and
biotechnology that have provided currently approved therapeutic agents
generally fell into two broad areas: recombinant DNA (rDNA) technology and
hybridoma techniques (to produce monoclonal antibodies). The technology that is
fueling our ever-widening under-standing of human cellular function and disease
processes is undergoing an explosive evolution. A wealth of additional and
innovative biotechnologies, have been, and will continue to be, developed in
orderto harvest the information found in the human genome. These technological
advances will provide a better understanding of the relationship between
genetics and biological function, unravel the underlying causes of disease,
explore the association of genomic variation and drug response, enhance
pharmaceutical research, and fuel the discovery and development of new and
novel biopharmaceuticals. These revolutionary tech-nologies and additional
biotechnology-related techni-ques are improving the very competitive and costly
process of drug development of new medicinal agents, diagnostics and medical
devices. Some of the technol-ogies and techniques described in this chapter are
both well established and commonly used applications of biotechnology producing
potential therapeutic pro-ducts now in development including clinical trials.
Still more applications are evolving as you read this text. Their full impact
on the future of molecular medicine has yet to be imagined.
No meaningful discussion of pharmaceutical biotechnology and 21st
century healthcare can occur without mention of “omics” technologies. Research
in genomics and proteomics is heralded as the next important supply source of
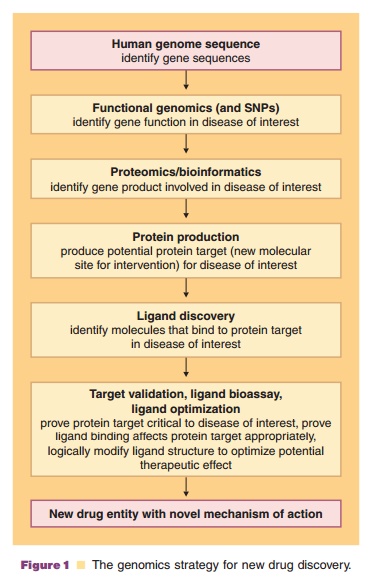
innovative future drug design targets. With the Human Genome Project (HGP)
rapidly approaching closure, researchers are turning increasingly to the task
of converting the DNA sequence data into information that will potentially
improve, and perhaps even revolutionize, drug discovery (Fig. 1) and
pharmaceutical care. Pharmaceutical scientists are poised to take advantage of
this scientific breakthrough by incorporating state-of-the-art genomics and
proteomics techniques along with the associated technologies utilized in
bioinfor-matics, metabonomics/metabolomics, systems biology, pharmacogenomics,
toxicogenomics, glycomics, and chemical genomics into a new drug discovery,
development, and clinical translation paradigm.
Techniques such as genetically engineered ani-mals (including transgenic animals, transgenic plants, and knockout mice), protein engineering, peptide chemistry and peptidomimetics, cell therapy and regenerative medicine are directly influencing the pharmaceutical sciences and are well positioned to impact significantly modern medical and pharma-ceutical care. These additional techniques in biotech-nology and molecular biology are being rapidly exploited to bring new drugs to market.
It is not the intention of this author to detail each and every
biotechnology technique exhaustively, since numerous specialized resources
already meet that need. Rather, this chapter will illustrate and enumer-ate
various biotechnologies that should be of key interest to pharmacy students,
practicing pharmacists, and pharmaceutical scientists because of their effect
on many aspects of pharmacy.
Related Topics