Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Ozone: Laboratory preparation, Properties, Uses, structure, layer
Ozone
Ozone is an allotropic form of oxygen and its molecular formula is O3.
It is an unstable dark blue diamagnetic gas. The presence of ozone in extremely
small quantities has been observed in the atmosphere in places near the seaside
(or) big lakes. It is present in sufficient quantities in the atmosphere at
attitudes of 12 to 15 miles above the earth's surface. Ozone is particularly
important since there is a layer of ozone in the upper atmosphere which absorbs
harmful UV radiations from the sun and protect the people and other living
organisms on the earth.
Laboratory preparation
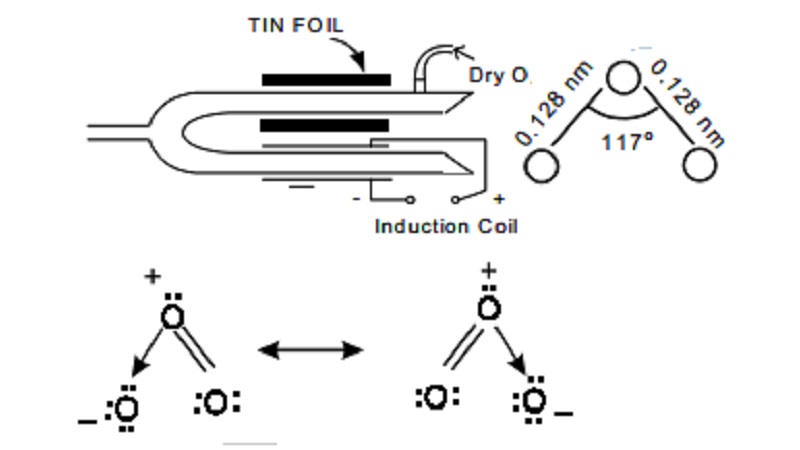
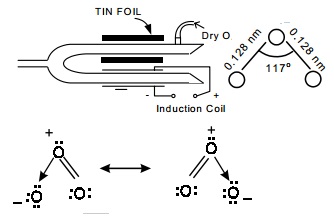
Ozone is prepared in the laboratory by passing
silent electrical discharges through dry oxygen in an apparatus known as the
ozoniser. The commonly used ozoniser is Siemen's ozoniser
(i)
Siemen's ozoniser
It
consists of two concentric metal tubes sealed together at one end. The inner
side of the inner tube and the outer side of the outer tube are coated with tin
foil and connected to one terminal each of an induction coil. A current of pure
dry oxygen at low temperature is passed through annular space between the two
tubes and by the silent action of electric discharge, the oxygen is partially
converted into ozone. The sample of gas escaping from ozoniser is called
ozonised oxygen and contains about 12% ozone.
Properties
(i) Physical properties
It is a light blue gas which condense at 160.6 K
into a dark blue liquid. This liquid freezes at 23.3 K.
Chemical properties
1) Decomposition : Pure ozone decomposes with an explosive violence.
2) Oxidising
action : Since it can liberate an atom of nascent oxygen easily (O3 -- > O2 + O)
it acts as a powerful xidizing agent.
i)Lead sulphide is oxidised to lead sulphate PbS + 4O3 -- > PbSO4 +
4O2
ii) Potassium
manganate is xidized to potassium permanganate
2K2MnO4 + H2O + O3 -- > 2KmnO4 + 2KOH + O2
3) Ozone
reacts with peroxides and reduces it to oxides with the liberation of oxygen.
BaO2 + O3 -- > BaO + 2O2
H2O2 + O3 -- > H2O + 2O2
Uses of ozone
1.
It is used as germicide and disinfectant.
2.
It is used for bleaching oils, ivory, flour,
starch, etc.
3.
Used in the manufacture of artificial silk and
synthetic camphor.
Ozone structure
The ozone molecule consists of three
oxygen atoms having a bent structure
Each O atom contributes six valence electrons
and so the total 3x6=18 electrons.
Ozone molecule is said to be resonance hybrid of
the two contributing forms I & II.

Ozone layer
Ozone is produced in the upper atmosphere through absorption of a
SKRWRQ K# RI XOWUDYLROHW OLJKW E\ DQ 22 molecule.
O2(g) K# -- > 2O(g)
O(g) + O2(g) -- > O3(g)
The ozone molecule formed has an excess of
energy and dissociates back to O2 and O and it reacts with another molecule (M)
such as CO2, N2 or O2, which causes the excess energy thus stabilizes the ozone
molecule
O*3(g) + M(g) -- > O3(g)+ M*(g)
Factors affecting ozone layer
The ozone in the upper atmosphere is important in shielding us from the
intense ultraviolet radiation coming from the sun. The so-called ozone shield
is a shell about 30 km altitude which contains enough ozone to absorb short
wavelength UV radiation (less than 300 nm). Hence ozone is considered to be
'earth's protective umbrella'. The absorption causes dissociation of O3 to
reform O2.
O3(g) K# -- > O2(g) + O(g)
2O(g) -- > O·2(g)
O·2(g) + M(g) -- > O2(g)+ M·(g)
Existence of ozone shield owes to the life on the earth, since living
tissues are very sensitive to wavelengths of ultraviolet absorbed by ozone. In
recent years, the shield is damaged mainly by supersonic aircraft and chlorofluorocarbon
products in the jet exhaust reduce ozone, and decreases its concentration in
the shield.
Chlorofluorocarbons react with O3 and causes a hole in the
ozone layer. CFC's are used as refrigerants and as propellants in some
"aerosol sprays". The lifetime of CFCs are so long that in another
decades, the extent of ozone depletion in the upper atmosphere will be
tremendous. It is reported that the holes caused in the ozone layer over the
Antarctic and Arctic ocean are due to the use of CFCs in aerosols and
refrigerators. It is feared that this will allow an excessive amount of UV
light to reach the earth which will cause skin cancer (melanoma) in human.
UV
CFC -- > Cl(g)
Cl(g) + O3(g) -- > ClO(g) + O2(g)
ClO + O(g) -- > Cl(g) + O2(g)
O3(g) + O(g) -- > 2O2(g)
It is also
seen that the oxides of nitrogen (from car exhausts) and the halogen can damage
the ozone layer. Therefore the protecting shield of the earth must be protected
by taking immediate steps over the control of pollution.
Related Topics