Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Oxy-Acids of Phosphorus: Preparation, properties, Structure
Oxy-Acids of Phosphorus
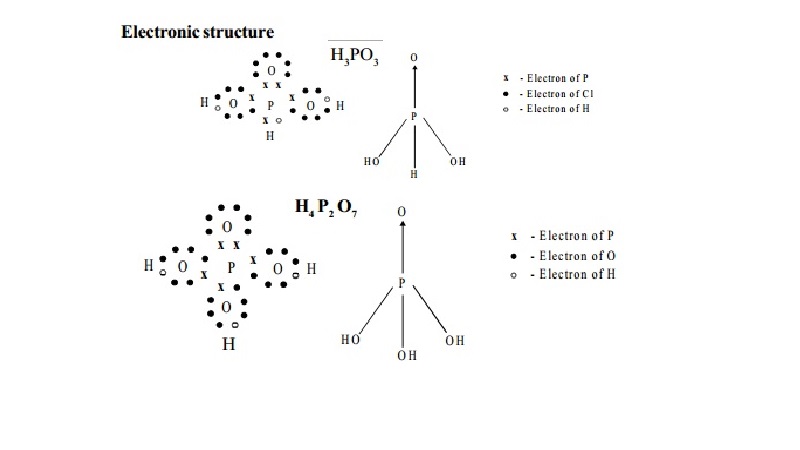
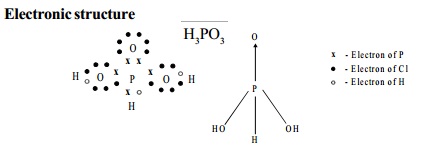
I. Phosphorus acid - H3PO3
It is prepared by the action of cold water on phosphorus
(III) oxide or phosphorus (III)
chloride.
P2O3 + 3H2O ® 2H3PO3
PCl3 + 3H2O ® H3PO3 + 3HCl
Physical properties
It is a white crystalline solid with garlic taste.
Chemical Properties
1. Acidic nature: It is a dibasic acid and gives salts of two types.
H3PO3 + NaOH ® NaH2PO3 + H2O
Sodium dihydrogen Phosphite
H3PO3 + 2NaOH ®
Na2HPO3 + 2H2O
Disodium hydrogen Phosphite
2.
When it is heated it undergoes auto-oxidation and reduction to form
phosphoric acid and phosphine.
D
4H3 PO3 ® 3H3PO4 + PH3
3. It is a powerful reducing agent because it has P-H
bond. It reduces silver
nitrate solution into silver.
2AgNO3 + H3PO3 + H2O ® 2Ag +H3PO4+2HNO3
Electronic structure
Use: It is used
as a reducing agent
II. Ortho phosphoric Acid, H3PO4
Preparation
1. It is prepared by dissolving phosphorus pentoxide in water and boiling the solution.
P2O5 + 3H2O ® 2H3PO4
2. Laboratory preparation: In the laboratory
orthophosphoric acid can be
prepared by boiling a mixture of red phosphorus with 50%
nitric acid in a flask fitted with a
reflux condenser on a water bath till no more oxides of nitrogen are liberated.
Iodine acts as a catalyst. The product is evaporated
below 453 K and then
cooled in a vaccum desiccator surrounded by freezing
mixture when crystals of orthophosphoric
acid are deposited.
P+5HNO3 ® H3PO4 +5NO2 +H2O
Physical properties
1. It is a deliquescent crystalline solid.
2. It is soluble in water.
Chemical properties
1. It is a tribasic acid. It combines with alkalies like
NaOH to form three series
of salts.
H3PO4 +NaOH ® NaH2PO4 + H2O
Sodium Di hydrogen Phosphate
H3PO4 +2NaOH ® Na2HPO4 + 2H2O
Disodium hydrogen Phosphate
H3PO4 + 3NaOH ® Na3PO4 + 3H2O
Sodium Phosphate
2.
On heating it gives pyrophosphoric acid at 523 K and at 589 K gives
metaphosphoric acid
523K 589K
H3PO4 H4 P2O7 2HPO3 + H2O
3. On reaction with silver nitrate, it gives yellow
precipitate of silver phosphate.
H3PO4 + 3AgNO3 ® Ag3PO4+3HNO3
Uses
1. It is used in the preparation of HBr and HI as a
substitute for sulphuric acid.
2. It is used as souring agent in the preparation of soft
drinks.
3. It is used in the preparation of phosphate salts of
sodium, potassium and ammonium.
4.
It is used in the
manufacture of phosphatic fertilisers.
Structure
Being a tribasic acid, the structure of phosphoric acid
is represented as
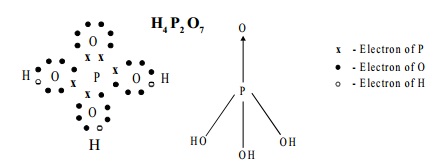
III. B. Pyrophosphoric acid, H4 P2 O7
Preparation:
Pyrophosphoric acid is prepared by heating orthophosphoric
acid to 523 K - 533 K.
2H3PO4 ® H4P2O7 + H2O
Physical Properties
It is a colourless crystalline solid.
Chemical Properties
1. It is reconverted to orthophosphoric acid on
boiling with water
H4P2O7 + H2O ® 2H3PO4
2. When heated strongly, it yields metaphosphoric
acid
H4P2O7 2HPO3 + H2O
Structure:
The Structure of pyrophosphoric acid is represented as:
Related Topics