Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Magnesium: Important Ores, Metallurgy, Properties, Uses
Magnesium
The magnesium comes from the name of the mineral magnesite, which in
turn is believed to stem from the name Magnesia. The British chemist Humphry
Davy discovered the pure element magnesium in 1808.
Due to its low density, it is considered to be a structural unit.
Important Ores
Magnesium does not occur in the native state. In
the combined state it occurs very abundantly in the earth crust.
Magnesite, MgCO3 Dolomite,
MgCO3, CaCO3
Epsomsalt, MgSO4, 7H2O Carnallite MgCl2 KCl.6H2O
However magnesium ion Mg2+, is the third most abundant
dissolved ion in the oceans, after Cl- and Na+. The
oceans are the best sources for magnesium. It is widely distributed in the
vegetable kingdom being present in chlorophyll, the green colouring matter of
the leaves.
Metallurgy
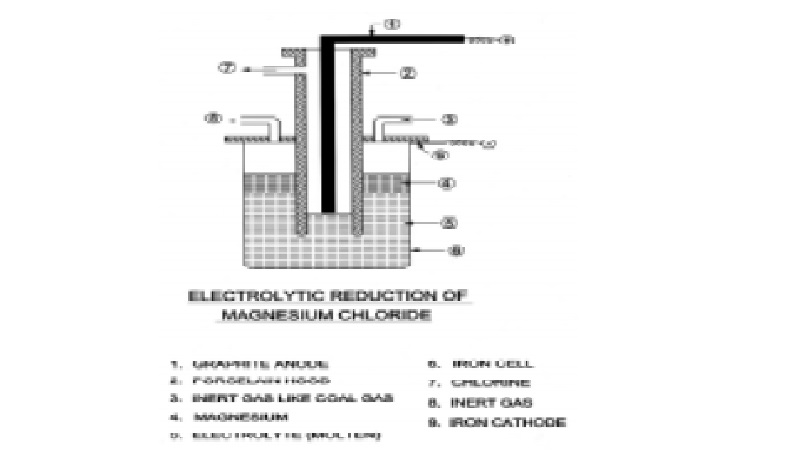
Magnesium is prepared on a large scale by the electrolysis of either
fused magnesium chloride or magnesia.
1. Electrolysis of fused
magnesium chloride
The purified carnallite ore is the principal source for this process. A
mixture of equal quantities of carnallite and NaCl is fused to a clear liquid
at 973K. The alkali chloride prevents hydrolysis of magnesium chloride and
increases the conductivity of the fused mass.
The electrolysis of the fused mass is carried
out in an atmosphere of coal gas in air tight iron cell which can hold 6-7
tonnes of the electrolyte. The temperature of the elctrolyte bath is maintained
at 970K. The iron cell itself acts as a cathode unlike the anode consists of a
carbon or graphite rod surrounded by a porcelain tube through which the
liberated chlorine escapes. Molten magnesium being lighter than the
electrolyte, rises to the surface and is periodically removed with perforated
ladle. The electrolysis is carried out in an atmosphere of coal gas so as to
avoid the oxidation of molten magnesium. The metal thus obtained is 99.9% pure.
It may be further purified by remelting with a flux of anhydrous magnesium
chloride and sodium chloride.
Physical
Pure magnesium metal is a relatively active
silvery white metal. At slightly below its melting point, it is malleable and
ductile and can be drawn into wire or rolled into ribbon in which form it is
generally sold. It is a very light metal.
Chemical Properties
1.Action of Air : It does not tarnish in dry air but a layer of white oxide is formed on its surface in moist air.
2.With air on burning : It burns in air or oxygen with a dazzling light rich in ultraviolet rays, forming magnesium oxide and magnesium
nitride.
2Mg + O2 -- > 2Mg O
3Mg + N2 -- > Mg3N2
3.With CO2
It continues to burn in CO2,
2 Mg + CO2 -- > 2 MgO + C
4.Action of Water
When heated with steam it burns brilliantly
producing magnesium oxide and hydrogen.
Mg H 2O
-- > MgO H 2
steam
5. Action of Acids
Dilute HCl or H2SO4 gives hydrogen with
magnesium. With dilute HNO3, part of the hydrogen liberated is oxidised by
nitric acid, which itself is reduced to a variety of products depending upon
the concentration. With concentrated HNO3, it gives ammonium nitrate.
4Mg + 10HNO3 -- > 4 Mg(NO3)2 + NH4NO3 + 3H2O
6. Displacement of Metals
It is a strongly electropositive metal and hence
Mg displaces nearly all the metals from the solutions of their salts eg.
Mg + 2AgNO3 -- > Mg(NO3)2+ 2Ag
7. Reducing Action
Mg has great affinity for oxygen and it
liberates sodium, potassium, boron and silicon from their oxides at high
temperatures.
K2O + Mg -- > MgO + 2K
B2O3 + 3Mg -- > 3MgO + 2B
Uses of Magnesium
1.
In flashlight photography, pyrotechnics and in
fireworks.
2.
As a reducing agent in the preparation of boron
and silicon and deoxidiser in metallurgy.
Related Topics