Chapter: Medical Immunology: Hypersensitivity Reactions
Immune Complex–Induced Hypersensitivity Reactions (Type III Hypersensitivity)
IMMUNE COMPLEX–INDUCED HYPERSENSITIVITY REACTIONS (TYPE III HYPERSENSITIVITY)
In the course of acute or chronic infections, or as a consequence of the production of autoantibodies, antigen-antibody complexes (also known as Immune complexes) are likely to be formed in circulation or in tissues to which the pertinent self antigens or microbial anti-gens are expressed or have been adsorbed. Both scenarios can lead to inflammatory changes which are characteristic of the so-called immune complex diseases .
Circulating immune complexes are usually adsorbed to red cells and cleared by the phagocytic system. In most cases this will be an inconsequen-tial sequence of events, but in cases where there is massive formation of circulating im-mune complexes (e.g., serum sickness), the clearance capacity of the phagocytic system is exceeded, and inflammatory reactions can be triggered by the deposition of those immune complexes in tissues. A simplified sequence of events leading to immune complex–induced inflammation is shown in Figure 20.1.
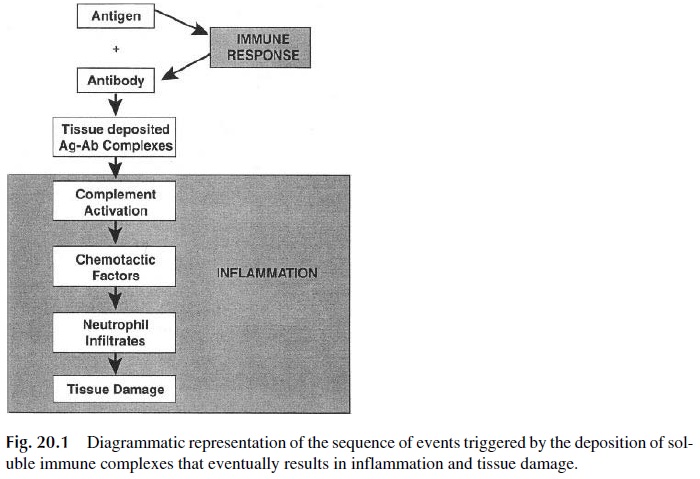
The in situ formation of immune complexes is likely to be a scenario more likely involved in the pathogenesis of inflammatory reactions. The adsorption of circulating anti-gens of microbial origin or released by dying cells to a variety of tissues seems to be a relatively common event. If the same antigens trigger a humoral immune response, immune complex formation may take place in the tissues where the antigens are adsorbed, in which case clearance by the phagocytic system may become impossible. In fact, tissue-bound im-mune complexes are very strong activators of the complement system and of phagocytic cells, triggering a sequence of events leading to tissue inflammation virtually identical to that observed in cases of in situ immune reactions involving tissue antigens and the corresponding antibodies.
A. The Arthus Reaction
Arthus, who observed that the intradermal injection of antigen into an animal previously sensitized results in a local inflammatory reaction, first described this reaction at the turn of the century. A human equivalent of this reaction can be observed in some reactions to immunization boosters in individuals who have already reached high levels of immunity.
The Arthus reaction is triggered by the combination of complement-fixing IgG antibodies (characteristically predominating in hyperimmune states in most species) and tissue-fixed antigens. The lag time between antigen challenge and the reaction is usually 6 hours, which is considerably longer than the time lag of an immediate hypersensitivity reaction, but considerably shorter than that of a delayed hypersensitivity reaction.
Arthus reactions are typically elicited in the skin. They are usually edematous in the early stages, but later can become hemorrhagic and, eventually, necrotic. Deep tissues can also be affected, because the same pathogenic mechanisms can lead to deep tissue inflam-mation whenever the antigen, although intrinsically soluble, is unable to diffuse freely and remains retained in or around its penetration point (e.g., the perialveolar spaces for inhaled antigens).
Because it is easily induced in a variety of laboratory animals, the Arthus reaction is one of the best studied models of immune complex disease. Immunohistological studies have shown that soon after antigen is injected in the skin, IgG antibody and C3 will appear in perivascular deposits at the site of injection. This is followed by a massive influx of gran-ulocytes, believed to result from activation of the complement system by the in situ–formed immune complexes (ICs). The importance of granulocytes was confirmed in experiments in which investigators tried to induce the Arthus reaction in laboratory animals rendered neutropenic by administration of nitrogen mustard or of antineutrophil serum. Under these experimental conditions the inflammatory reaction is prevented and the reactions does not develop.
In spite of their pathogenic role, granulocytes will actively engulf and catabolize the tissue-deposited ICs, eliminating the trigger for the inflammatory reaction. As the ICs are eliminated, the cellular infiltrate changes from a predominance of neutrophils and other granulocytes to a predominance of mononuclear cells, which is usually associated with the healing stage. The degree of healing depends on whether the exposure to the triggering anti-gen is a discrete event or repeated over time. Single or widely spaced exposure is usually followed by complete healing, while frequently repeated exposures tend to lead to irre-versible damage.
B. Serum Sickness
In the preantibiotic era, the treatment of rabies, bacterial pneumonia, diphtheria, and other infections involved the administration of heterologous antisera as a way to transfer immu-nity to the offending agents. In many instances, serotherapy appeared to be successful and the patient improved, but a week to 10 days after the injection of heterologous antiserum, the patient developed what was termed as “serum sickness”: a combination of cutaneous rash (often purpuric), fever, arthralgias, mild acute glomerulonephritis, and carditis. Cur-rently, serum sickness as a complication of passive immunotherapy with heterologous antisera is seen after injection of heterologous antisera to snake venom, after the adminis-tration of mouse monoclonal antibodies in cancer immunotherapy, and after the adminis-tration of heterologous (monoclonal or polyclonal) antilymphocyte sera in transplanted pa-tients. But it can also be a side effect of some forms of drug therapy, particularly with penicillin and related drugs.
Serum sickness is extremely easy to reproduce in experimental animals through the injection of heterologous proteins. Basically two types of experimental serum sickness can be induced:
· Acute, after a single immunization with a large dose of protein
· Chronic, after repeated daily injections of small doses of protein
While acute serum sickness is reversible, the chronic form, which closely resembles human glomerulonephritis, is usually associated with irreversible damage.
In all types of serum sickness, the initial event is the triggering of a humoral immune response, which explains the lag period of 7–10 days between the injection of heterologous protein (or drug) and the beginning of clinical symptoms. The lag period is shorter and the reaction more severe if there has been presensitization to the antigen in question.
As soon as antibodies are produced in sufficient amounts, they combine with the anti-gens (which at that time are still present in relatively large concentrations in the serum of the injected individual or experimental animal). Initially, the antigen-antibody reaction will take place in conditions of great antigen excess, and the resulting complexes are too small to activate complement or to be taken up by phagocytic cells and will remain in circulation without major consequences. As the immune response progresses and increasing amounts of antibody are produced, the antigen-antibody ratio will be such that intermediate-sized immune complexes will be formed. The intermediate-sized immune complexes are poten-tially pathogenic; they are large enough to activate complement and small enough to cross the endothelial barrier (particularly if vascular permeability is increased as a consequence of complement activation and release of C3a and C5a). Once they reach the extravascular space, inflammatory cells are recruited and activated and initiate the chain of events lead-ing to tissue inflammation. As in the case of the Arthus reaction, the inflammatory changes associated with serum sickness do not take place or are very mild if complement or neu-trophils are depleted.
The deposition of immune complexes can take place in different organs, such as the myocardium (causing myocardial inflammation), skin (causing erythematous rashes), joints (causing arthritis), and kidney (causing acute glomerulonephritis). Soluble immune complexes can also be absorbed by formed elements of the blood, particularly erythrocytes, neutrophils, and platelets. Although red cell absorption is usually a protective mechanism , if the amounts and characteristics of red blood cell–absorbed ICs are such that the regulatory function of CR1 is overridden, hemolysis may take place. Thrombocy- topenia and neutropenia can also result from the activation of the complement system by cell-associated immune complexes. Purpuric rashes due to thrombocytopenia are frequently seen in serum sickness.
Related Topics