Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
High-Performance Liquid Chromatography (HPLC): Stationary Phases
Stationary Phases
In liquid–liquid chromatography the stationary phase
is a liquid film coated
on a packing material consisting of 3–10
ÎĽm porous silica
particles. The stationary phase may be partially soluble in the
mobile phase, causing
it to “bleed” from the
column over time. To prevent this loss of stationary phase,
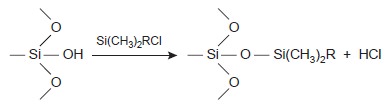
it is covalently bound to the sil- ica particles. Bonded stationary phases are attached by reacting the silica particles with an organochlorosilane of the general
form Si(CH3)2RCl, where
R is an alkyl or substituted alkyl group.
To prevent unwanted interactions between the solutes and any
unreacted –SiOH groups, the silica frequently is “capped” by reacting it with
Si(CH3)3Cl; such columns are designated as end-capped.
The
properties of
a stationary
phase are determined by the nature of the organosilane’s alkyl
group. If R is a polar functional group, then the stationary
phase will be polar. Examples
of polar stationary phases include those for which R
contains a cyano (–C2H4CN), diol (–C3H6OCH2CHOHCH2OH), or amino
(–C3H6NH2) functional group. Since
the stationary phase
is polar, the mobile phase is
a nonpolar or moderately polar
solvent. The combination of a polar
stationary phase and a nonpolar mobile
phase is called
normal-phase chromatography.
In reverse-phase chromatography, which is the more
commonly encountered form of HPLC, the
stationary phase is nonpolar and
the mobile phase
is polar. The most common nonpolar stationary phases use an organochlorosilane for which the R
group is an n-octyl
(C8) or n-octyldecyl (C18) hydrocarbon chain. Most
reverse- phase separations are carried out using a buffered aqueous
solution as a polar mo- bile
phase. Because the silica substrate is subject to hydrolysis in basic solutions, the pH of the mobile phase
must be less than 7.5.
Related Topics