Chapter: Organic Chemistry: Structure and bonding
sp3 Hybridization
SP3 HYBRIDIZATION
Key Notes
Definition
In sp3 hybridization, the s and the p orbitals of the second shell are ‘mixed’ to form four hybridized sp3 orbitals of equal energy.
Electronic configuration
Each
hybridized orbital contains a single unpaired electron and so four bonds are
possible.
Geometry
Each sp3 orbital is shaped like a
deformed dumbbell with one lobe much larger than the other. The hybridized
orbitals arrange themselves as far apart from each other as possible such that
the major lobes point to the cor-ners of a tetrahedron. sp3 Hybridization explains the tetrahedral carbon in
saturated hydrocarbon structures.
Sigma bonds
Sigma (σ) bonds are strong bonds
formed between two sp3
hybridized car-bons or between ansp3
hybridized carbon and a hydrogen atom. Aσ bond formed between two sp3 hybridized carbon atoms involves the overlap of half
filled sp3 hybridized
orbitals from each carbon atom. Aσ bond formed between an sp3 hybridized carbon and a hydrogen atom involves a
half-filled sp3 orbital
from carbon and a half-filled 1s
orbital from hydrogen.
Nitrogen, oxygen and chlorine
Nitrogen,
oxygen, and chlorine atoms can also be sp3
hybridized in organic molecules. This means that nitrogen has three half-filled
sp3 orbitals and can form
three bonds which are pyramidal in shape. Oxygen has two half-filled sp3orbitals and can form two
bonds which are angled with respect to eachother. Chlorine has a single
half-filled sp3 orbital
and can only form a single bond. All the bonds which are formed are σ bonds.
Definition
In sp3
hybridization, the 2s orbital is
mixed with all three of the 2p
orbitals to give a set of four sp3 hybrid orbitals. (The number of hybrid
orbitals must equal the number of original atomic orbitals used for mixing.)
The hybrid orbitals will each have the
same energy but will be
different in energy
from the original atomic orbitals.
That energy difference
will reflect the
mixing of the
respect- ive atomic orbitals. The
energy of each hybrid orbital is greater than the original s orbital but less than the original p orbitals (Fig. 1).
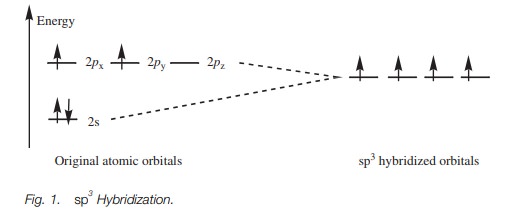
Electronic configuration
The valence electrons for carbon can now be fitted into the sp3 hybridized orbitals (Fig. 1). There was a total of four electrons in the original 2s and 2p orbitals. The s orbital was filled and two of the p orbitals were half filled. After hybridization,there is a total of four hybridized sp3 orbitals all of equal energy. By Hund’s rule, they are all half filled with electrons which means that there are four unpaired electrons. Four bonds are now possible.
Geometry
Each of the sp3
hybridized orbitals has the same shape – a rather deformed looking dumbbell (Fig. 2).
This deformed dumbbell looks more like a p orbital than an s orbital
since more p orbitals were involved
in the mixing process.
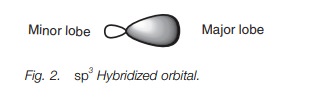
Each sp3
orbital will occupy a space as far apart from each other as possible by
pointing to the corners of a tetrahedron (Fig.
3). Here, only the major lobe of each hybridized orbital has been shown and
the angle between each of these lobes is 109.5 . This is what is meant by the
expression tetrahedral carbon. The
three-dimensional shape of the tetrahedral carbon can be represented by drawing
a nor-mal line for bonds in the plane of the page. Bonds going behind the page
are represented by a hatched wedge, and bonds coming out the page are
represented by a solid wedge.
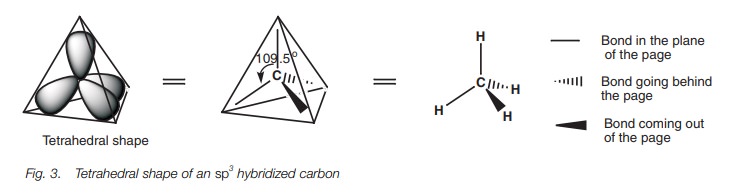
Sigma bonds
A half-filled sp3 hybridized orbital from one carbon atom can be used
to form a bond with a half-filled sp3
hybridized orbital from another carbon atom. In Fig. 4a, the major lobes of the two sp3 orbitals overlap directly leading to a strong σ bond. It is the ability of hybridized orbitals to form strong σ bonds that explains why hybridization takes place in the first
place. The deformed dumbbell shapes allow a much better orbital overlap than
would be obtained from a pure s
orbital or a pure p orbital. A σ bond between an sp3
hybridized carbon atom and a hydrogen atom involves the carbon atom using one
of its half-filled sp3
orbitals and the hydrogen atom using its half-filled 1s orbital (Fig. 4b).
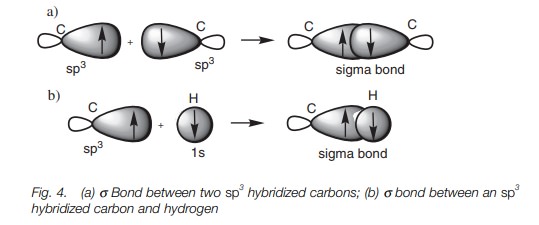
Nitrogen, oxygen, and chlorine
Nitrogen,
oxygen and chlorine
atoms can also
be sp3
hybridized in organic structures. Nitrogen has five valence
electrons in its second shell. After hybridization, it will have three half-filled
sp3 orbitals and can form
three bonds. Oxygen has six valence electrons. After hybridization, it will
have two half-filled sp3
orbitals and will form two bonds. Chlorine has seven valence electrons. After
hybridization, it will have one half-filled sp3
orbital and will form one bond.
The four sp3
orbitals for these three atoms form a tetrahedral arrangement with one or more
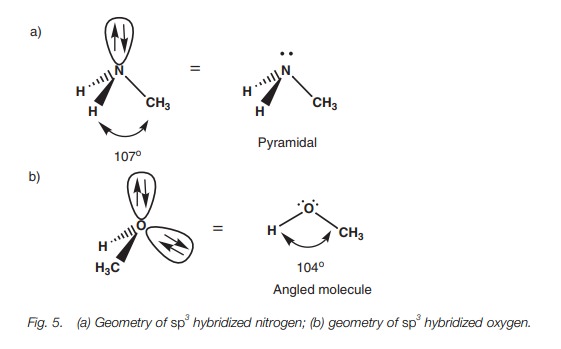
of the orbitals occupied by a lone pair of electrons. Considering the atoms
alone, nitrogen forms a pyramidal shape where the bond angles are slightly less
than 109.5° (c. 107°) (Fig. 5a).
This compression of the bond angles is due to the orbital containing the lone
pair of electrons, which demands a slightly greater amount of space than a
bond. Oxygen forms an angled or bent shape where two lone pairs of electrons
compress the bond angle from 109.5° to c.
104 (Fig. 5b).
Alcohols, amines, alkyl halides, and ethers all
contain sigma bonds involving nitrogen, oxygen, or chlorine. Bonds between
these atoms and carbon are formed by the overlap of half-filled sp3 hybridized orbitals from
each atom. Bonds involv-ing hydrogen atoms (e.g. O–H and N–H) are formed by the
overlap of the half-filled 1s orbital
from hydrogen and a half-filled sp3
orbital from oxygen or nitrogen.
Related Topics