Chapter: Plant Biochemistry: Photosynthesis is an electron transport process
Water is split by photosystem II
Water is split by photosystem II
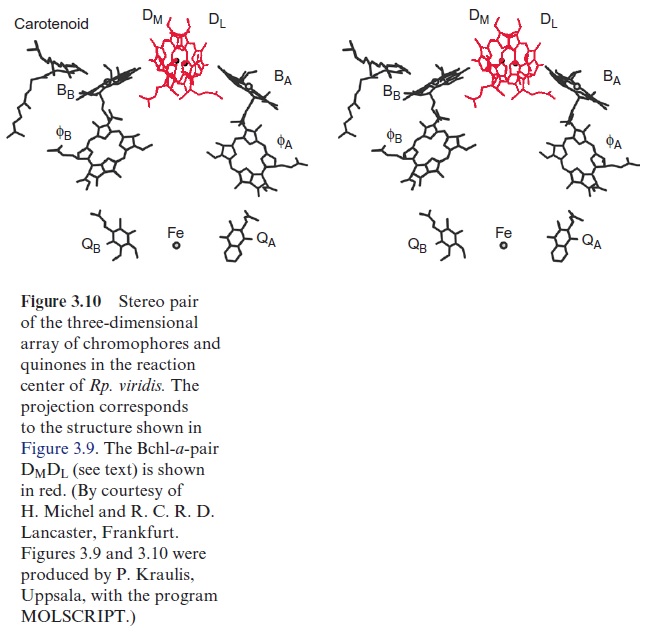
The groups of Horst Witt and Wolfgang Saenger (both in Berlin) resolved the three-dimensional structure of PS II by X-ray structure analysis of crystals from the PS II of the thermophilic cyanobacteria Thermosynechococcus elongatis. The subsequent X-ray structure analysis of PS I revealed that PS II and PS I are constructed after the same basic principles as the reactiocenters of purple bacteria . This, and sequence analyses, clearly demonstrate that all these photosystems have a common origin. Thus PS II also has a chl-a pair in the center, although the distance between the two molecules is so large that probably only one of the two chl-a molecules reacts with the exciton. Two arms, each with one chl-a and one pheophy-tin molecule, are connected with this central pair as in the purple bacteria shown in Figure 3.10. Also in the cyanobacteria, only one of these arms appears to be involved in the electron transport.
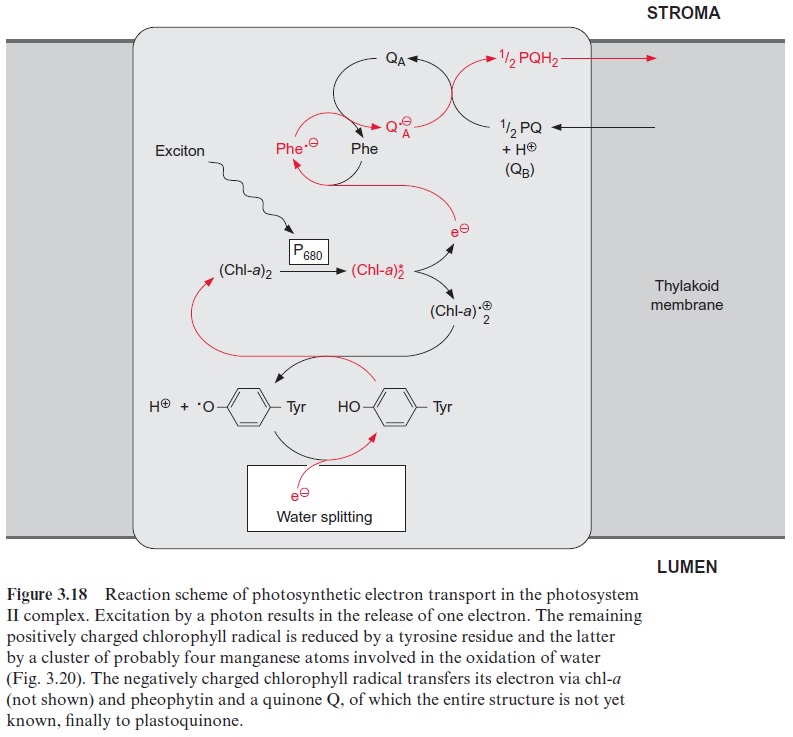
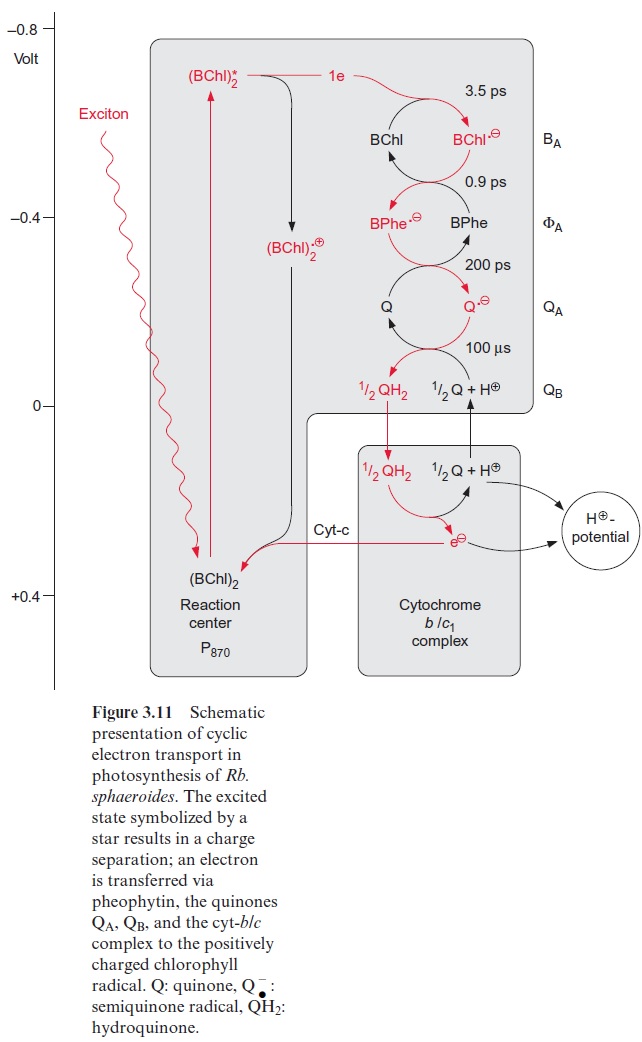
In contrast to the bacterial reaction center the excitation of the reaction center results in an electron transfer via the chl-a monomer to pheophytin (Phe), and from there to a tightly bound plastoquinone (QA), thus forming a semiquinone radical (Fig. 3.18). The electron is then further transferred to a loosely bound plastoquinone (QB). This plastoquinone (PQ) (Fig. 3.19) accepts two electrons and two protons one after the other and is thus reduced to hydroquinone (PQH2). The hydroquinone is released from the photosynthesis complex and may be regarded as the final product of photosystem II. This sequence, consisting of a transfer of a single electron between (chl-a)2 and QAand the transfer of two electrons between QA and QB, corresponds to the reaction sequence shown for Rb. sphaeroides (Fig. 3.11). The only difference is that the quinones are ubiquinone or menaqui-none in bacteria and plastoquinone in photosystem II.
However, the similarity between the reaction sequence in PS II and the photosystem of the purple bacteria applies only to the electron acceptor region. The electron donor function in PS II of plants is completely differ-ent from that in purple bacteria. The electron deficit in (chl-a)2+ • caused by non-cyclic electron transport is compensated for by electrons derived from the oxidation of water. In the transport of electrons from water to chloro-phyll manganese cations and a tyrosine residue are involved. The (chl-a)2+ • radical with a redox potential of about +1.1 volt is such a strong oxidant that it can withdraw an electron from a tyrosine residue in the protein of the reaction center and a tyrosine radical remains. This reactive tyrosine residue is often designated as Z. The electron deficit in the tyrosine radical is restored by oxidation of a manganese ion (Fig. 3.20). The PS II com-plex contains several manganese ions, probably four, which are close to each other. This arrangement of Mn ions is called the Mn cluster. The Mn cluster depicts a redox system that can take up and release four electrons. During this process the Mn ions probably change between the oxidation state Mn3+ and Mn4+ .
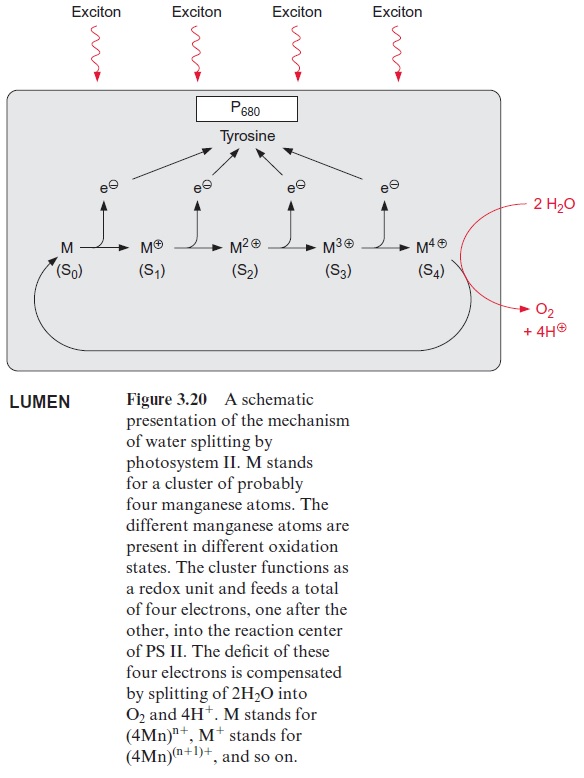
To liberate one molecule of O2 from water, the reaction center must with-draw four electrons and thus capture four excitons. The time differences between the capture of the single exciton in the reaction center depends on the intensity of illumination. If oxidation of water were to proceed stepwise, oxygen radicals could be formed as intermediary products, especially at low light intensities. Oxygen radicals have a destructive effect on biomolecules such as lipids and proteins . The water splitting machinery of the Mn clusters minimizes the formation of oxygen radical intermediates by supplying the reaction center via tyrosine with four electrons one after the other (Fig. 3.20). The Mn cluster is transformed during this transfer from the ground oxidation state stepwise to four different oxidation states (these have been designated as S0and S1–S4).
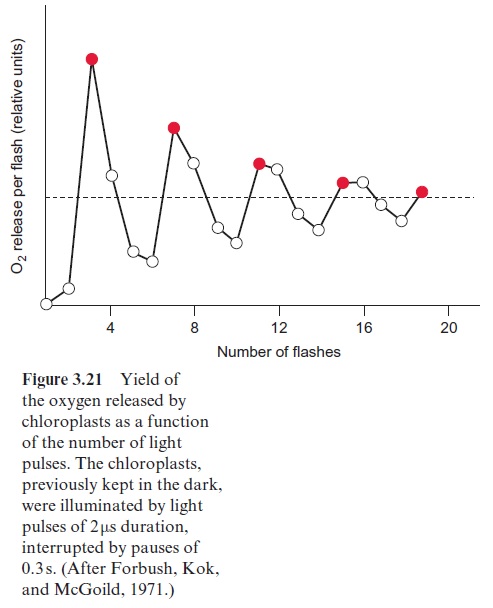
Experiments by Pierre Joliot (France) and Bessel Kok (USA) presented evidence that the water splitting apparatus can be in five different oxida-tion states (Fig. 3.21). When chloroplasts that were kept in the dark were then illuminated by a series of light pulses, an oscillation of the oxygen release was observed. Whereas after the first two light pulses almost no O2 was released, the O2 release was maximal after three pulses and then after a further four pulses, and so on. An increasing number of light pulses, how-ever, dampened the oscillation. This can be explained by pulses that do not cause excitation of PS II and thus desynchronize the oscillation. In dark-ened chloroplasts the water splitting apparatus is in the S1 state. After the fourth oxidation state (S4) has been reached, O2 is released in one reaction and the Mn cluster returns to its ground oxidation state (S0). During this reaction, protons from water are released to the lumen of the thylakoids. The formal description of this reaction is:
2 H2O → 4H+ + 2O2-
2 O2- + M4+ → O2 + M
Figuratively speaking, the four electrons needed in the reaction center are loaned in advance by the Mn cluster and then repaid at one stroke by oxidizing water to synthesize one oxygen molecule. In this way the Mn clus-ter minimizes the formation of oxygen radicals in photosystem II. Despite this safety device, still some oxygen radicals are formed in the PS II com-plex which damage the proteins of the complex.
Photosystem II complex is very similar to the reaction center in purple bacteria
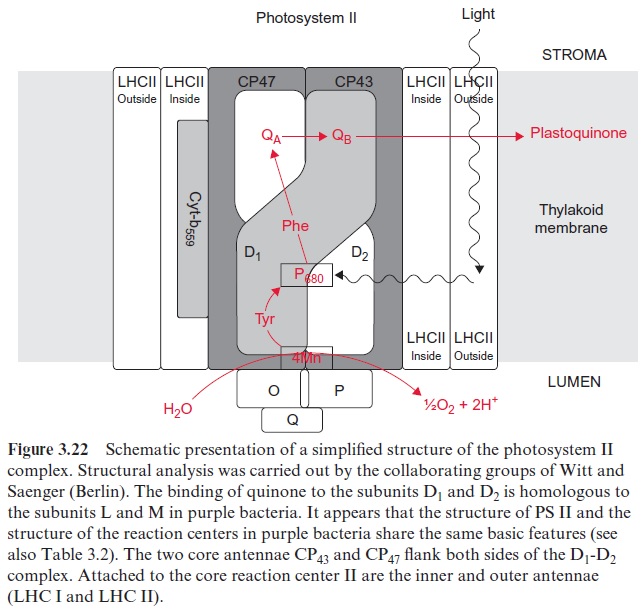
The center of the PS II complex is a heterodimer consisting of the sub-units D1 and D2 with six chl-a, two pheophytin, two plastoquinone, and one to two carotenoid molecules bound to it. The D1 and D2 proteins are homologous to each other and also to the L proteins and M proteins from the reaction center of the purple bacteria . As in purple bacteria, only the pheophytin molecule bound to the D1 protein of PS II is involved in electron transport. QA is bound to the D2 protein, whereas QB is bound to the D1 protein. The Mn cluster is probably enclosed by both the D1 and D2 proteins. The tyrosine that is reactive in electron transfer is a constituent of D1. The subunits O, P, Q stabilize the Mn cluster. The two subunits CP 43 and CP 47 (CP means chlorophyll protein) each bind about 15 chloro-phyll molecules and form the core complex of the antenna shown in Figure 2.10. CP 43 and CP 47 flank both sides of the D1-D2 complex. Cyt-b559 does not seem to be involved in the electron transport of PS II; possibly its func-tion is to protect the PS II complex from light damage. The inner and outer light harvesting complexes of LHC II are arranged at the periphery.
The D1 protein of the PS II complex has a high turnover; it is constantly being resynthesized. It seems that the D1 protein wears out during its func-tion, perhaps through damage by oxygen radicals, which still occurs despite all the protection mechanisms. It has been estimated that the D1 protein is replaced after 106 to 107 catalytic cycles of the PS II reaction center.
A number of compounds that are similar in their structure to plasto-quinone can block the plastoquinone binding site at the D1 protein, caus-ing inhibition of photosynthesis. Such compounds are used as weed killers (herbicides). Before the effect of these compounds is discussed in detail, some general aspects of the application of herbicides shall be introduced.
Mechanized agriculture usually necessitates the use of herbicides
About 50% of the money spent worldwide for plant protection is expended for herbicides. The high cost of labor is one of the main reasons for using herbicides in agriculture. It is cheaper and faster to keep a field free of weeds by using herbicides rather than manual labor. Weed control in agriculture is necessary not only to decrease harvest losses by weed competition, but also because weeds hinder the operation of harvesting machineries; fields free of weeds are a prerequisite for a mechanized agriculture. The herbicides usu-ally block a specific reaction of the plant metabolism and have a low toxic-ity for animals and humans. A large number of herbicides inhibit photosystem II by being antagonists to plastoquinone. To achieve substantial inhibition the herbicide molecule has to bind to most of the many photosynthetic reaction centers. To be effective, 125 to 4,000 g of these herbicides have to be applied per hectare.
In an attempt to reduce the amount of herbicides applied to the soil, new efficient herbicides have been developed that inhibit key biosynthetic processes such as the synthesis of fatty acids, amino acids, carotenoids, or chlorophyll. There are also herbicides that act as analogues of phytohormones or mitosis inhibitors. Some of these herbi-cides are effective with amounts as low as 5 g per hectare.
Some herbicides are taken up only by the roots and others by the leaves. To keep the railway tracks free of weeds, nonselective herbicides are employed, which destroy the complete vegetation. Nonselective herbicides are also used in agriculture, e.g., to combat weeds in citrus plantations. In the latter case, herbicides are applied that are only taken up by the leaves to combat herbaceous plants at the ground level. Especially interesting are selective herbicides that combat only weeds and effect cultivars as little as possible. Selectivity can be due to different uptake efficien-cies of the herbicide in different plants, different sensitivities of the metabo-lism towards the herbicide, or different ability of the plants to detoxify the herbicide. Important mechanisms that plants utilize to detoxify herbicides and other foreign compounds (xenobiotics) are the introduction of hydroxyl groups by P-450 monooxygenases and the formation of glu-tathione conjugates . Selective herbicides have the advantage that weeds can be destroyed at a later growth stage of the cultivars where the dead weeds form a mulch layer conserving water and preventing erosion.
In some cases, the application of herbicides has led to the evolution of herbicide-resistant plant mutants. Conventional breeding has used such mutated plants to generate herbicide-resistant cultivars. In contrast to the occurrence of herbicide resistance by accidental mutations, nowadays genetic engineering is employed on a very large scale to generate cultivars which are resistant to a certain herbicide, allowing weed control in the presence of the growing cultivar.
A large number of herbicides inhibit photosynthesis: the urea deriva-tive DCMU (Diuron, DuPont), the triazine Atrazine (earlier Ciba Geigy), Bentazon (BASF) (Fig. 3.23), and many similar compounds function as herbicides by binding to the plastoquinone binding site on the D1 pro-tein and thus blocking the photosynthetic electron transport. Nowadays, DCMU is not often used, as the required dosage is high and its degradation is slow. It is, however, often used in the laboratory to inhibit photosynthe-sis in an experiment (e.g., of leaves or isolated chloroplasts). Atrazine acts selectively: maize plants are relatively insensitive to this herbicide since they have a particularly efficient mechanism for its detoxification . Because of its relatively slow degradation in the soil, the use of Atrazine has been restricted in some countries, e.g., Germany. In areas where cer-tain herbicides have been used continuously over the years, some weeds have become resistant to these herbicides. In some cases, the resistance can be traced back to mutations resulting in a single amino acid change in the D1-proteins. These changes do not markedly affect photosynthesis of these weeds, but they do decrease binding of the herbicides to the D1-protein.
Related Topics