Chapter: 11th Chemistry : UNIT 4 : Hydrogen
Water - Compounds of Hydrogen
Compounds of Hydrogen
Water
Water is one of the most abundant compounds of hydrogen
and our earth’s surface contains approximately 70 % of ocean which is the major
source of water. However, sea water contains many dissolved salts hence it can
not be used directly. Water is essential for all living things and our body
contains about 65% water.
Ortho-H2O and Para-H2O
Water exists in the interstellar clouds, in
proto-planetary disks, in the comets and icy satellites of the solar system. In
particular, the ortho-to-para ratio (OPR) of water in space has recently
received attention. Like hydrogen, water can also be classified into ortho-H2O,
in which the spin directions of the nuclei of the hydrogen atoms are parallel,
and para-H2O, in which the directions are antiparallel. At the
temperature conditions of the earth (300 K), the OPR of H2O is 3.
However, at low temperatures (< 50 K) the amount of para-H2O
increases. It is known that the OPR of water in interstellar clouds and comets
has more para-H2O (OPR = 2.5) than on Earth.
Physical Properties:
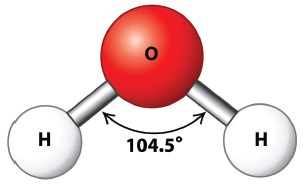
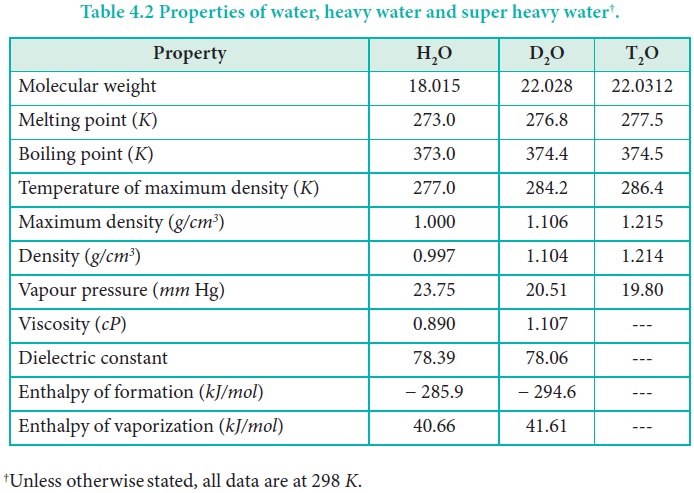
Water is a colourless and volatile liquid. The peculiar properties of water in the condensed phases are due to the presence of inter molecular hydrogen bonding between water molecules. Hydrogen bonding is responsible for the high melting and boiling points of water. Some of the physical parameters of water are listed in Table 4.2.
Chemical
Properties:
Water reacts with metals, non-metals and other compounds
differently. The most reactive metals are the alkali metals. They decompose
water even in cold with the evolution of hydrogen leaving an alkali solution.
2Na + 2 H2O → 2 NaOH + H2
The group 2 metals (except beryllium) react in a similar
way but less violently. The hydroxides are less soluble than those of Group 1.
Ba + 2H2O → Ba(OH)2 + H2
Some transition metals react with hot water or steam to
form the corresponding oxides. For example, steam passed over red hot iron
results in the formation of iron oxide with the release of hydrogen.
3Fe + 4H2O → Fe3O4 + H2
Lead and copper decompose water only at a white heat.
Silver, gold, mercury and platinum do not have any effect on water. In the
elemental form, the non-metals such as carbon, sulphur and phosphorus normally
do not react with water. However, as we have seen earlier, carbon will react
with steam when it is red (or white) hot to give water gas.
On the other hand, the halogens react with water to give
an acidic solution. For example, chlorine forms hydrochloric acid and hypo
chlorous acid. It is responsible for the antibacterial action of chlorine
water, and for its use as bleach.
Cl2 + H2O → HCl + HOCl
Fluorine reacts differently to liberate oxygen from water.
2F2 +
2 H2O → 4HF + O2
In a similar way, compounds of non-metals react with water
to give acidic or alkaline solutions. For example, solutions of carbonates are
slightly alkaline.
CO32− + H2O → HCO3− + OH−
Water is an amphoteric oxide. It has the ability to accept
as well as donate protons and hence it can act as an acid or a base. For
example, in the reaction with HCl it accepts proton where as in the reaction
with weak base ammonia it donates proton.
NH3 + H2O → NH4+
+ OH−
HCl + H2O → H3O+ + Cl−
Water dissolves ionic compounds. In addition, it also
hydrolyses some covalent compounds.
SiCl4 + 2 H2O → SiO2 + 4
HCl
P4O10 + 6 H2O → 4 H3PO4
Many salts crystallized from aqueous solutions form
hydrated crystals. The water in the hydrated salts may form co-ordinate bond or
just present in interstitial positions of crystals.
Examples: [Cr(H2O)6]Cl3 –
All six water molecules form co-ordinate bond
BaCl2.2H2O – Both the water
molecules are present in interstitial positions.
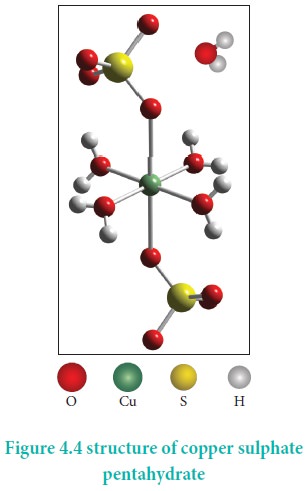
CuSO4.5H2O – In this compound four
water molecules form co-ordinate bonds while the fifth water molecule, present
outside the co-ordination, can form intermolecular hydrogen bond with another
molecule. [Cu(H2O)4]SO4.H2O
Hard and Soft Water:
Hard water contains high amounts of mineral ions. The most
common ions found in hard water are the soluble metal cations such as magnesium
& calcium, though iron, aluminium, and manganese may also be found in
certain areas. Presence of these metal salts in the form of bicarbonate,
chloride and sulphate in water makes water ‘hard’. When hard water is boiled
carbonates of magnesium and calcium present in it gets precipitated. On the
other hand, water free from soluble salts of calcium and magnesium is called
soft water. The hardness of water is of two types, viz., temporary hardness and
permanent hardness.
Temporary Hardness and its removal:
Temporary hardness is primarily due to the presence of
soluble bicarbonates of magnesium and calcium. This can be removed by boiling
the hard water followed by filtration. Upon boiling, these salts decompose into
insoluble carbonate which leads to their precipitation. The magnesium carbonate
thus formed further hydrolysed to give insoluble magnesium hydroxide.
Ca(HCO3)2 → CaCO3 + H2O
+ CO2
Mg(HCO3)2 → MgCO3 + H2O
+ CO2
MgCO3 + H2O → Mg(OH)2 +
CO2
The resulting precipitates can be removed by filtration.
Alternatively, we can use Clark’s method in which,
calculated amount of lime is added to hard water containing the magnesium and
calcium, and the resulting carbonates and hydroxides can be filtered-off.
Ca(HCO3)2 + Ca(OH )2 → 2CaCO3
+ 2H2O
Mg (HCO3)2 + 2 Ca(OH )2 →
2CaCO3 + Mg(OH)2
+2 H2O
Permanent Hardness:
Permanent hardness of water is due to the presence of
soluble salts of magnesium and calcium in the form of chlorides and sulphates
in it. It can be removed by adding washing soda, which reacts with these metal
(M = Ca or Mg) chlorides and sulphates in hard water to form insoluble
carbonates.
MCl2 + Na2CO3 → MCO3
+ 2 NaCl
MSO4 + Na2CO3 → MCO3
+ Na2SO4
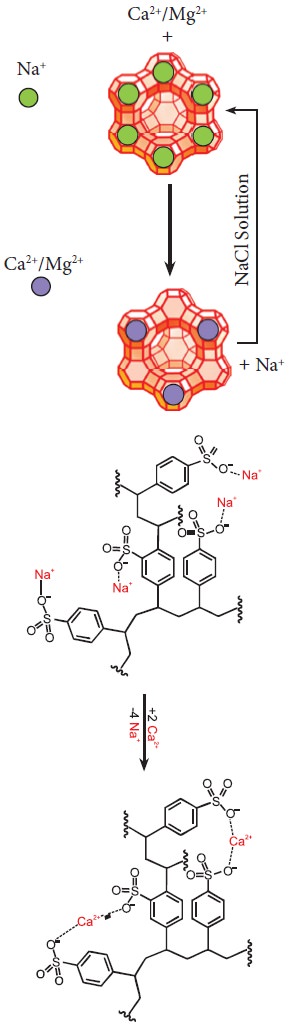
In another way to soften the hard water is by using a
process called ion-exchange. That is, hardness can be removed by passing
through an ion-exchange bed like zeolites or column containing ion-exchange
resin. Zeolites are hydrated sodium alumino-silicates with a general formula,
NaO∙Al2O3∙xSiO2∙yH2O (x = 2 to 10, y = 2 to 6).
Zeolites have porous structure in which the monovalent sodium ions are loosely
held and can be exchanged with hardness producing metal ions (M = Ca or Mg) in
water. The complex structure can conveniently be represented as Na2-Z
with sodium as exchangeable cations.
Na2-Z + M2+ → M-Z+ 2 Na+
When exhausted, the materials can be regenerated by
treating with aqueous sodium chloride. The metal ions (Ca2+ and Mg2+)
caught in the zeolite (or resin) are released and they get replenished with
sodium ions.
M-Z + 2NaCl → Na2-Z + MCl2
Related Topics