Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Van't Hoff Equation
Van't
Hoff Equation
This
equation gives the quantitative temperature dependence of equilibrium constant
(K). The relation between standard free energy change (ΔG°) and equilibrium
constant is
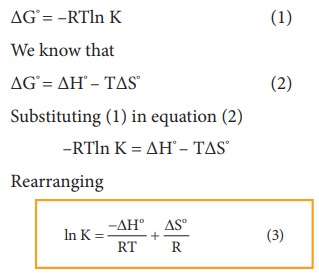
Differentiating
equation (3) with respect to temperature,
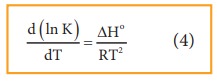
Equation
4 is known as differential form of van’t Hoff equation.
On
integrating the equation 4, between T1 and T2 with their
respective equilibrium constants K1 and K2.
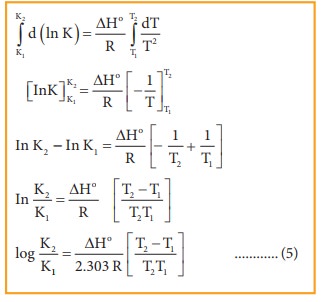
Equation 5 is known as integrated form of van’t Hoff equation.
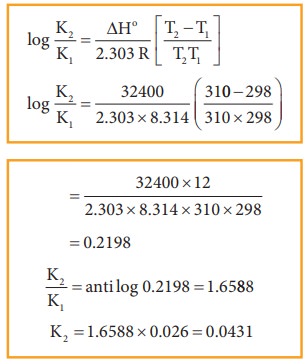
Problem
For
an equilibrium reaction Kp = 0.0260 at 25° C ΔH= 32.4 kJmol-1,
calculate Kp at 37° C
Solution:
T1=25
+ 273 = 298 K
T2
= 37 + 273 = 310 K
ΔH
= 32.4 KJmol-1 = 32400 Jmol-1
R=8.314
JK-1 mol-1
KP1=
0.0260
Kp2=?
Related Topics