Chapter: Medical Surgical Nursing: Management of Patients With Structural, Infectious, and Inflammatory Cardiac Disorders
Valvuloplasty
Valve Repair and Replacement Procedures
VALVULOPLASTY
The
repair, rather than replacement, of a cardiac valve is referred to as valvuloplasty. The type of
valvuloplasty depends on the cause and type of valve dysfunction. Repair may be
made to the commissures between the leaflets in a procedure known as com-missurotomy, to the annulus of the
valve by annuloplasty, to theleaflets, or to the chordae by chordoplasty.
Most
valvuloplasty procedures require general anesthesia and often require
cardiopulmonary bypass. Some procedures, how-ever, can be performed in the
cardiac catheterization laboratory; these procedures do not always require
general anesthesia or cardio-pulmonary bypass. Percutaneous partial cardiopulmonary
bypass is used in some cardiac catheterization laboratories. The
cardio-pulmonary bypass is achieved by inserting a large catheter (ie, cannula)
into two peripheral blood vessels, usually a femoral vein and an artery. Blood
is diverted from the body through the venous catheter to the cardiopulmonary
bypass machine and returned to the
patient through the arterial catheter.
The
patient is usually managed in a critical care unit for the first 24 to 72 hours
after surgery. Care focuses on hemodynamic stabilization and recovery from
anesthesia. Vital signs are assessed every 5 to 15 minutes and as needed until
the patient recovers from anesthesia or sedation and then every 2 to 4 hours
and as needed. Intravenous medications to increase or decrease blood pressure
and to treat dysrhythmias or altered heart rates are ad-ministered, and their
effects are monitored. The intravenous medications are gradually decreased
until they are no longer required or the patient takes needed medication by
another route (eg, oral, topical). Patient assessments are conducted every 1 to
4 hours and as needed, with particular attention to neurologic, respiratory,
and cardiovascular assessments.
After
the patient has recovered from anesthesia and sedation, is hemodynamically
stable without intravenous medications, and assessments are stable, the patient
is usually transferred to a telemetry or surgical unit for continued
postsurgical care and teaching. The nurse provides wound care and patient
teaching regarding diet, activity, medications, and self-care. Patients are
discharged from the hospital in 1 to 7 days. In general, valves that have
undergone valvuloplasty function longer than replace-ment valves, and the
patients do not require continuous anti-coagulation.
Commissurotomy
The
most common valvuloplasty procedure is commissurotomy. Each valve has leaflets;
the site where the leaflets meet is called the commissure. The leaflets may adhere to one another and close
thecommissure (ie, stenosis). Less commonly, the leaflets fuse in such a way
that, in addition to stenosis, the leaflets are also pre-vented from closing
completely, resulting in a backward flow of blood (ie, regurgitation). A
commissurotomy is the procedure performed to separate the fused leaflets.
CLOSED COMMISSUROTOMY
Closed
commissurotomies do not require cardiopulmonary by-pass. The valve is not
directly visualized. The patient receives a general anesthetic, a midsternal
incision is made, a small hole is cut into the heart, and the surgeon’s finger
or a dilator is used to break open the commissure. This type of commissurotomy
has been performed for mitral, aortic, tricuspid, and pulmonary valve disease.
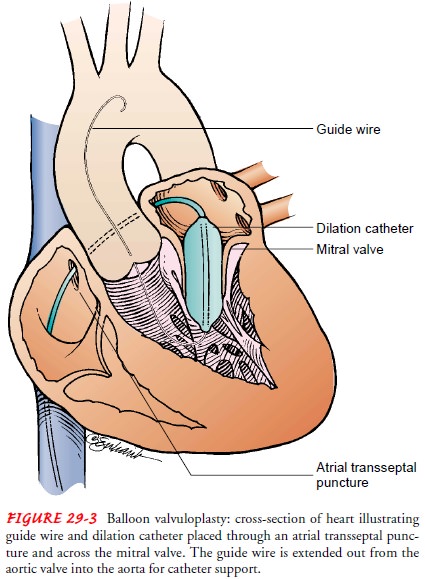
Balloon Valvuloplasty.
Balloon valvuloplasty (Fig. 29-3) is anothertype of closed
commissurotomy beneficial for mitral valve stenosis in younger patients, for
aortic valve stenosis in elderly patients, and for patients with complex
medical conditions that place them at high risk for the complications of more
extensive surgical proce-dures. Most commonly used for mitral and aortic valve
stenosis, balloon valvuloplasty also has been used for tricuspid and pulmonic
valve stenosis. The procedure is performed in the cardiac catheter-ization
laboratory, and the patient may receive a local anesthetic. Patients remain in
the hospital 24 to 48 hours after the procedure.
Mitral
valvuloplasty is contraindicated for patients with left atrial or ventricular
thrombus, severe aortic root dilation, signif-icant mitral valve regurgitation,
thoracolumbar scoliosis, rotation of the great vessels, and other cardiac
conditions that require open heart surgery.
Mitral balloon valvuloplasty involves advancing one or two catheters into the right atrium, through the atrial septum into the left atrium, across the mitral valve into the left ventricle, and out into the aorta. A guide wire is placed through each catheter, and the original catheter is removed. A large balloon catheter is then placed over the guide wire and positioned with the balloon across the mitral valve. The balloon is then inflated with a dilute angio-graphic solution. When two balloons are used, they are inflated simultaneously. The advantage of two balloons is that they are each smaller than the one large balloon often used, making smaller atrial septal defects. As the balloons are inflated, they usually do not completely occlude the mitral valve, thereby permitting some forward flow of blood during the inflation period.
All
patients have some degree of mitral regurgitation after the procedure. Other
possible complications include bleeding from the catheter insertion sites,
emboli resulting in complications such as strokes, and rarely, left-to-right
atrial shunts through an atrial septal defect caused by the procedure.
Aortic
balloon valvuloplasty also may be performed by passing the balloon or balloons
through the atrial septum, but it is per-formed more commonly by introducing a
catheter through the aorta, across the aortic valve, and into the left
ventricle. The one-balloon or the two-balloon technique can be used for
treating aortic stenosis. The aortic procedure is not as effective as the
pro-cedure for the mitral valve, and the rate of restenosis is nearly 50% in
the first 12 to 15 months after the procedure (Braunwald et al., 2001).
Possible complications include aortic regurgitation, emboli, ventricular
perforation, rupture of the aortic valve annulus, ven-tricular dysrhythmias,
mitral valve damage, and bleeding from the catheter insertion sites.
OPEN COMMISSUROTOMY
Open
commissurotomies are performed with direct visualization of the valve. The
patient is under general anesthesia, and a me-dian sternotomy or left thoracic
incision is made. Cardio-pulmonary bypass is initiated, and an incision is made
into the heart. A finger, scalpel, balloon, or dilator may be used to open the
commissures. An added advantage of direct visualization of the valve is that
thrombus may be identified and removed, calci-fications can be seen, and if the
valve has chordae or papillary muscles, they may be surgically repaired.
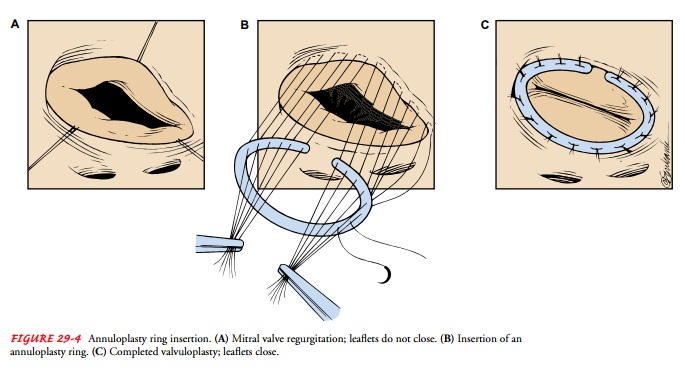
Annuloplasty
Annuloplasty is
the repair of the valve annulus (ie, junction ofthe valve leaflets and the
muscular heart wall). General anesthesia and cardiopulmonary bypass are
required for all annuloplasties. The procedure narrows the diameter of the
valve’s orifice and is useful for the treatment of valvular regurgitation.
There
are two annuloplasty techniques. One technique uses an annuloplasty ring (Fig.
29-4). The leaflets of the valve are sutured to a ring, creating an annulus of
the desired size. When the ring is in place, the tension created by the moving
blood and contracting heart is borne by the ring rather than by the valve or a
suture line, and progressive regurgitation is prevented by the repair. The
other technique involves tacking the valve leaflets to the atrium with su-tures
or taking tucks to tighten the annulus. Because the valve’s leaflets and the
suture lines are subjected to the direct forces of the blood and heart muscle
movement, the repair may degenerate more quickly than with the annuloplasty
ring technique.

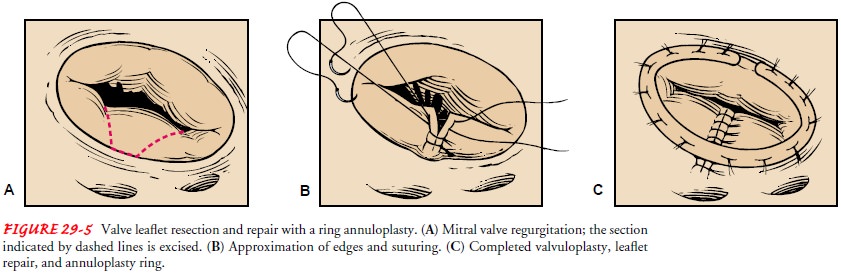
Leaflet Repair
Damage
to cardiac valve leaflets may result from stretching, short-ening, or tearing. Leaflet repair for elongated,
ballooning, or other excess tissue leaflets is removal of the extra tissue. The
elongated tissue may be folded over onto itself (ie, tucked) and sutured (ie,
leaflet plication). A wedge of tissue may be cut from the middle of the leaflet
and the gap sutured closed (ie., leaflet re-section) (Fig. 29-5). Short
leaflets are most often repaired by chordoplasty. After the short chordae are
released, the leaflets often unfurl and can resume their normal function of
closing the valve during systole. A piece of pericardium may also be sutured to
extend the leaflet. A pericardial patch may be used to repair holes in the
leaflets.
Chordoplasty
Chordoplasty is
the repair of the chordae tendineae. The mitralvalve is involved with
chordoplasty (because it has the chordae tendineae); seldom is chordoplasty
required for the tricuspid valve.
Regurgitation
may be caused by stretched, torn, or shortened chordae tendineae. Stretched
chordae tendineae can be shortened, torn ones can be reattached to the leaflet,
and shortened ones can be elongated. Regurgitation may also be caused by
stretched papillary muscles, which can be shortened.
Related Topics