Chapter: Modern Analytical Chemistry: Basic Tools of Analytical Chemistry
Units for Expressing Concentration
Units for
Expressing Concentration
Concentration is a general
measurement unit stating
the amount of solute present in a known amount of solution
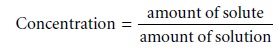
Although the terms “solute”
and “solution” are often associated with liquid sam- ples, they can be extended to gas-phase and solid-phase samples
as well. The actual
units for reporting concentration depend
on how the amounts of solute and solu-
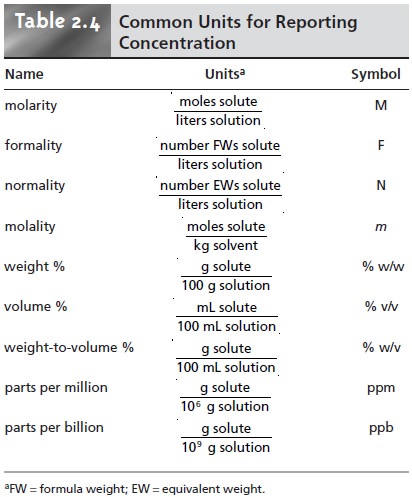
tion are measured. Table 2.4
lists the most
common units of concentration.
Molarity and Formality
Both molarity and formality express
concentration as moles
of solute per liter of solu-
tion. There is, however, a subtle difference between molarity and formality. Molarity
is the concentration of a particular chemical
species in solution. Formality, on
the other hand,
is a substance’s total concentration in solution without
regard to its spe-
cific chemical form. There is no difference between a substance’s molarity and for- mality if it dissolves without dissociating into
ions. The molar
concentration of a so- lution
of glucose, for
example, is the
same as its
formality.
For substances that ionize in solution, such as NaCl, molarity and formality are different. For example, dissolving 0.1 mol of NaCl in 1 L of water gives a solution containing 0.1 mol of Na+ and 0.1 mol of Cl–. The molarity of NaCl, therefore, is zero since there is essentially no undissociated NaCl in solution.
The solution, instead, is 0.1 M in Na+ and 0.1 M in Cl–. The formality of NaCl, however,
is 0.1 F because it represents the total amount
of NaCl in solution. The rigorous definition of molarity, for better
or worse, is largely ignored
in the current
literature, as it is in this
text. When we state that a solution
is 0.1 M NaCl we understand it to consist
of Na+ and Cl– ions. The unit of formality is used only when it provides a clearer de- scription of solution chemistry.
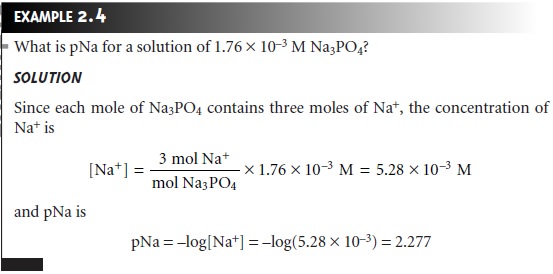
Molar concentrations are used so frequently that a symbolic
notation is often used to simplify its expression in equations and writing. The use of square brackets around a species indicates that we are referring to that species’
molar concentration. Thus, [Na+] is read as the “molar concentration of sodium ions.”
Normality
Normality is an older unit of concentration that, although once commonly used,
is frequently ignored in today’s laboratories. Normality is still
used in some
hand- books of analytical methods, and, for this reason,
it is helpful to understand its meaning. For example,
normality is the concentration unit used in Standard Meth-
ods for the Examination of Water and Wastewater,1 a
commonly used source
of ana- lytical methods
for environmental laboratories.
Normality makes use of the chemical
equivalent, which is the amount
of one chemical species
reacting stoichiometrically with another chemical species. Note that this definition makes
an equivalent, and thus normality, a function of the chemical reaction in which the species
participates. Although a solution of H2SO4
has a fixed molarity, its normality depends
on how it reacts.
The
number of equivalents, n, is
based on a reaction unit,
which is that
part of a chemical
species involved in a reaction. In a precipitation reaction, for example, the reaction unit is the charge
of the cation
or anion involved in the reaction; thus for the reaction
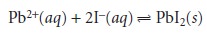
n = 2 for Pb2+ and n =
1 for I–. In an acid–base reaction, the reaction
unit is the number of H+ ions donated by an acid or accepted by a base.
For the reaction be- tween sulfuric acid and ammonia
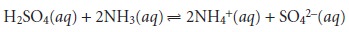
we
find that n =
2 for H2SO4
and n = 1 for NH3. For a complexation reaction, the
reaction unit is the number
of electron pairs
that can be accepted by the metal
or donated by the ligand. In the reaction
between Ag+ and NH3
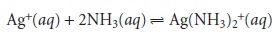
the
value of n for
Ag+ is
2 and that for NH3 is 1. Finally,
in an oxidation–reduction reaction
the reaction unit is the number of electrons released
by the reducing agent or accepted
by the oxidizing agent; thus,
for the reaction
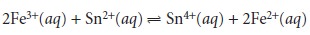
n =
1 for Fe3+ and n =
2 for Sn2+. Clearly, determining the number of equivalents
for a chemical species requires an understanding of how it reacts.
Normality is the number
of equivalent weights (EW)
per unit volume
and, like formality, is independent of speciation. An equivalent weight is defined
as the ratio of a chemical species’ formula
weight (FW) to the number
of its equivalents
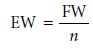
Consequently, the following simple relationship exists between normality and molarity.
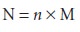
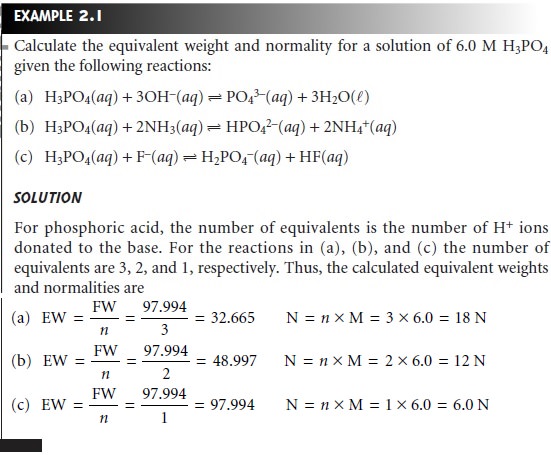
Example 2.1 illustrates the
relationship among chemical reactivity, equivalent weight, and normality.
Molality
Molality is
used in thermodynamic calculations where a temperature independent unit of concentration is needed. Molarity,
formality and normality
are based on the volume
of solution in which the solute is dissolved. Since
density is a temperature de- pendent property a solution’s volume,
and thus its
molar, formal and
normal concen- trations, will
change as a function of its temperature. By using the
solvent’s mass in place of its volume,
the resulting concentration becomes independent of temperature.
Weight, Volume, and Weight-to-Volume Ratios
Weight percent (% w/w), volume percent
(% v/v) and weight-to-volume percent (% w/v) express
concentration as units of solute per 100 units of sample. A solution
in which a solute has a concentration of 23% w/v contains
23 g of solute
per 100 mL of solution.
Parts per million (ppm) and parts
per billion (ppb)
are mass ratios
of grams of solute
to one million or one billion grams of sample,
respectively. For example,
a steel that is 450 ppm in Mn contains 450 μg of Mn for every gram of steel. If we approxi-
mate the density of an aqueous solution as 1.00 g/mL, then solution
concentrations can be
expressed in parts per million or parts per billion using the following
relationships.
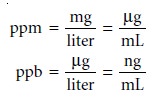
For gases a part per million usually
is a volume ratio. Thus, a helium concentration
of 6.3 ppm means that one liter
of air contains 6.3 μL of He.
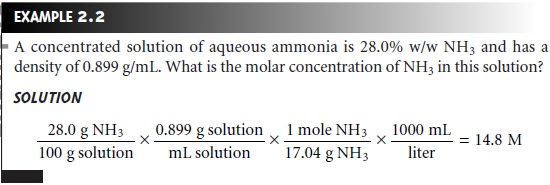
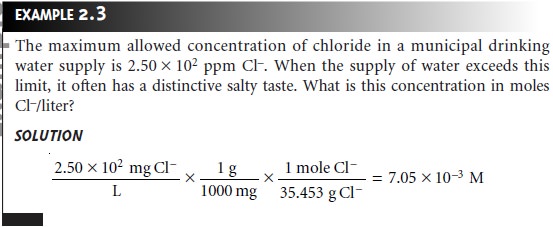
Converting Between Concentration Units
The units of concentration most frequently encountered in analytical chemistry are molarity, weight percent,
volume percent, weight-to-volume percent, parts per mil-
lion, and parts per billion. By recognizing the
general definition of concentration
given in equation 2.1, it is easy
to convert between
concentration units.
p-Functions
Sometimes it is inconvenient to use the concentration units in Table 2.4. For exam-
ple, during a reaction a reactant’s concentration may change by many orders
of mag- nitude. If we are interested in viewing the progress of the reaction
graphically, we might wish
to plot the
reactant’s concentration as a function of time or as a function
of the volume of a reagent being
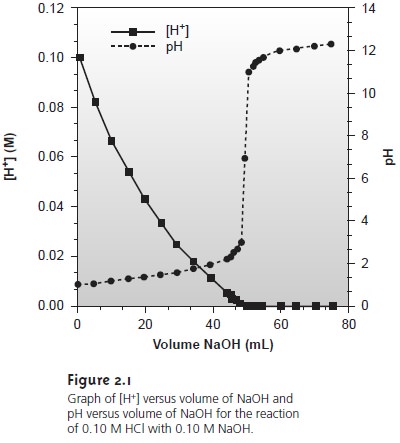
added to the reaction. Such is the case in Figure 2.1, where the molar concentration of H+ is
plotted (y-axis on left side of figure) as a
function of the volume of NaOH added
to a solution of HCl.
The initial [H+]
is 0.10 M, and its concentration after adding 75 mL of NaOH is 5.0 x 10–13 M. We can easily follow changes in the
[H+] over
the first 14 additions of NaOH. For
the last ten
addi- tions of NaOH,
however, changes in the [H+]
are too small
to be seen.
When working with concentrations that span many orders of magnitude, it is
often more convenient to express
the concentration as a p-function. The
p-func- tion of a number X is
written as pX and
is defined as
pX
= –log(X)
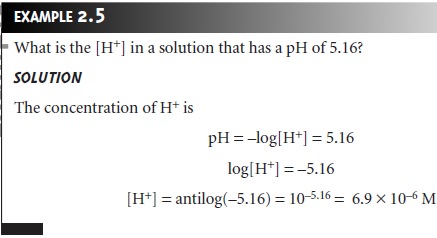
Thus, the pH of a solution that is 0.10 M H+ is
pH = –log[H+]
= –log(0.10) = 1.00
and the pH of 5.0 x 10–13 M H+ is
pH = –log[H+] = –log(5.0 x 10–13) = 12.30
Figure 2.1 shows
how plotting pH in place
of [H+] provides more detail
about how the concentration of H+ changes following the addition of NaOH.
Related Topics