Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Types of Thermodynamic systems
There are three types of thermodynamic systems depending on the nature of the boundary.
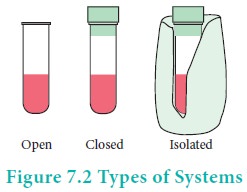
Isolated system:
A system which can exchange neither matter nor energy with its surroundings is called an isolated system. Here boundary is sealed and insulated. Hot water contained in a thermos flask, is an example for an isolated system. In this isolated system both energy (heat) and matter (water vapour) neither enter nor leave the system.
Closed system:
A system which can exchange only energy but not matter with its surroundings is called a closed system. Here the boundary is sealed but not insulated. Hot water contained in a closed beaker is an example for a closed system. In this system energy (heat) is transferred to the surroundings but no matter (water vapour) can escape from this system. A gas contained in a cylinder fitted with a piston constitutes a closed system.
Open system:
A System which can exchange both matter and energy with its surrounding is called an open system. Hot water contained in an open beaker is an example for open system. In this system both matter (water vapour) and energy (heat) is transferred to the surrounding.
All living things and chemical reactions are open systems because they exchange matter and energy with the surroundings.
Related Topics