Chapter: 12th Physics : Electromagnetic Waves
Types of Spectrum-Emission and Absorption Spectrum-Fraunhofer Lines
TYPES OF SPECTRUM-EMISSION AND ABSORPTION SPECTRUM-FRAUNHOFER
LINES
When an object burns, it
emits colours. That is, it emits electromagnetic radiation which depends on
temperature. If the object becomes hot then it glows in red colour. If the
temperature of the object is further increased then it glows in reddish-orange
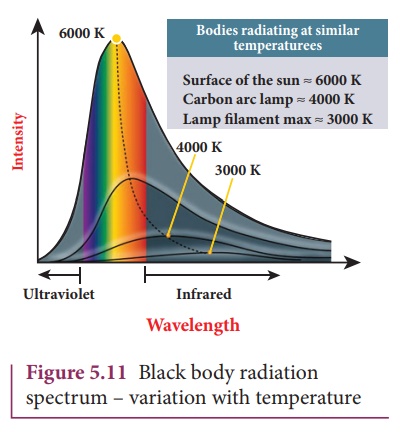
colour and becomes white when it is hottest. The spectrum in Figure 5.11
usually called as black body spectrum (refer plus one volume two Unit 8). It is
a continuous frequency (or wavelength) curve and depends on the body’s
temperature.
Suppose we allow a beam of white light to pass through the prism as shown in Figure 5.12, it is split into its seven constituent colours which can be viewed on the screen as continuous spectrum.

This phenomenon is known as dispersion of light and the definite pattern of colours
obtained on the screen after dispersion is called as spectrum. The plural for
spectrum is spectra. The spectra can be broadly classified into two catagories:
(a) Emission spectra
When the spectrum of
self luminous source is taken, we get emission spectrum. Each source has its
own characteristic emission spectrum. The emission spectrum can be divided into
three types:
(i) Continuous emission spectra (or continuous spectra)

If the light from
incandescent lamp (filament bulb) is allowed to pass through prism (simplest
spectroscope), it splits into seven colours. Thus, it consists of wavelengths
containing all the visible colours ranging from violet to red (Figure 5.13).
Examples: spectrum obtained from carbon arc, incandescent solids, liquids gives
continuous spectra.
(ii) Line emission spectrum (or line spectrum):

Suppose light from hot
gas is allowed to pass through prism, line spectrum is observed (Figure 5.14).
Line spectra are also known as discontinuous spectra. The line spectra are
sharp lines of definite wavelengths or frequencies. Such spectra arise due to
excited atoms of elements. These lines are the characteristics of the element
which means it is different for different elements. Examples: spectra of atomic
hydrogen, helium, etc.
(iii) Band emission spectrum (or band spectrum)
Band spectrum consists
of several number of very closely spaced spectral lines which overlapped
together forming specific bands which are separated by dark spaces, known as
band spectra. This spectrum has a sharp edge at one end and fades out at the
other end. Such spectra arise when the molecules are excited. Band spectrum is
the characteristic of the molecule hence, the structure of the molecules can be
studied using their band spectra. Examples, spectra of hydrogen gas, ammonia
gas in the discharge tube etc.
(b) Absorption spectra
When light is allowed to
pass through a medium or an absorbing substance then the spectrum obtained is
known as absorption spectrum. It is the characteristic of absorbing substance.
Absorption spectrum is classified into three types:
(i) Continuous absorption spectrum
When the light is passed
through a medium, it is dispersed by the prism, we get continuous absorption
spectrum. For instance, when we pass white light through a blue glass plate, it
absorbs everything except blue. This is an example of continuous absorption
spectrum.
(ii) Line absorption spectrum
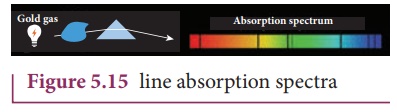
When light from the
incandescent lamp is passed through cold gas (medium), the spectrum obtained
through the dispersion due to prism is line absorption spectrum (Figure 5.15).
Similarly, if the light from the carbon arc is made to pass through sodium
vapour, a continuous spectrum of carbon arc with two dark lines in the yellow
region of sodium vapour is obtained.
(iii) Band absorption spectrum
When the white light is
passed through the iodine vapour, dark bands on continuous bright background is
obtained. This type of band is also obtained when white light is passed through
diluted solution of blood or chlorophyll or through certain solutions of
organic and inorganic compounds.
Fraunhofer lines
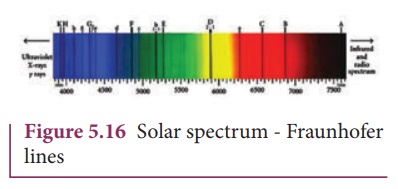
When the spectrum
obtained from the Sun is examined, it consists of large number of dark lines
(line absorption spectrum). These dark lines in the solar spectrum are known as
Fraunhofer lines (Figure 5.16). The absorption spectra for various materials
are compared with the Fraunhofer lines in the solar spectrum, which helps in
identifying elements present in the Sun’s atmosphere.
Related Topics