Chapter: 11th Chemistry : UNIT 1 : Basic Concepts of Chemistry and Chemical Calculations
Types of Redox Reactions
Types of Redox Reactions
Redox reactions are classified into the following types.
1. Combination reactions:
Redox reactions in which two substances combine to form a single compound are called combination reaction.
Example:
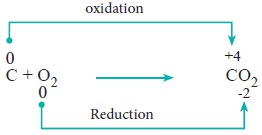
2. Decomposition reaction:
Redox reactions in which a compound breaks down into two or more components are called decomposition reactions. These reactions are opposite to combination reactions. In these reactions, the oxidation number of the different elements in the same substance is changed.
Example:

3. Displacement reactions:
Redox reactions in which an ion (or an atom) in a compound is replaced by an ion (or atom) of another element are called displacement reactions. They are further classified into (i) metal displacement reactions (ii) non-metal displacement reactions.
(i) Metal displacement reactions:
Place a zinc metal strip in an aqueous copper sulphate solution taken in a beaker. Observe the solution, the intensity of blue colour of the solution slowly reduced and finally disappeared.
The zinc metal strip became coated with brownish metallic copper. This is due to the following metal displacement reaction.

ii. Non-metal displacement
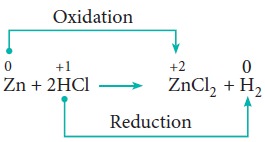
4. Disproportionation reaction (Auto redox reactions)
In some redox reactions, the same compound can undergo both oxidation and reduction. In such reactions, the oxidation state of one and the same element is both increased and decreased. These reactions are called disproportionation reactions.
Examples:
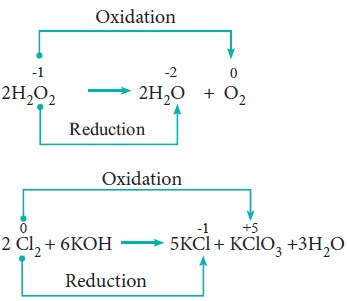
5. Competitive electron transfer reaction
In metal displacement reactions, we learnt that zinc replaces copper from copper sulphate solution. Let us examine whether the reverse reaction takes place or not. As discussed earlier, place a metallic copper strip in zinc sulphate solution. If copper replaces zinc from zinc sulphate solution, Cu2+ ions would be released into the solution and the colour of the solution would change to blue. But no such change is observed. Therefore, we conclude that among zinc and copper, zinc has more tendency to release electrons and copper to accept the electrons.
Let us extend the reaction to copper metal and silver nitrate solution. Place a strip of metallic copper in sliver nitrate solution taken in a beaker. After some time, the solution slowly turns blue. This is due to the formation of Cu2+ ions, i.e. copper replaces silver from silver nitrate. The reaction is,
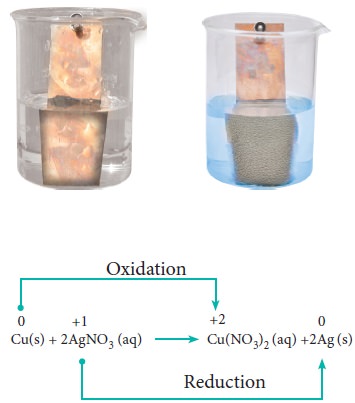
It indicates that between copper and silver, copper has the tendency to release electrons and silver to accept electrons.
From the above experimental observations, we can conclude that among the three metals, namely, zinc, copper and silver, the electron releasing tendency is in the following order.
Zinc > Copper > Silver
This kind of competition for electrons among various metals helps us to design (galvanic) cells. In XII standard we will study the galvanic cell in detail.
Related Topics