Chapter: Biology of Disease: Disorders of Acid Base Balance
Types of Acid–Base Disorders
TYPES OF ACID–BASE DISORDERS
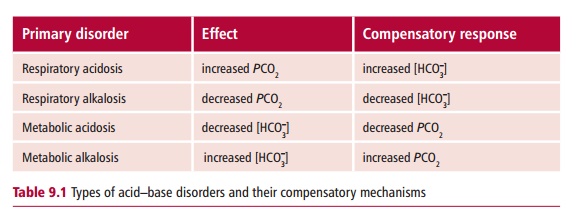
Disorders of acid–base balance are either acidoses or alkaloses. In an acidosis, there is accumulation of H+ in the blood and its pH falls below the reference range. In an alkalosis there is a depletion of H+ and therefore the blood has a pH above its reference range. Acid–base disorders can be further divided into two groups depending on their causes. If the abnormal pH occurs because of a metabolic or renal dysfunction, it is referred to as a metabolic acid–base disorder. When the abnormal pH is due to lung dysfunction, then it is a respiratory acid–base disorder. Physiological mechanisms that attempt to return the pH back to values within the reference range are referred to as compensation. Metabolic disorders cause a change in the concentration of HCO3– in the blood but respiratory disorders cause a change in its PCO2 (Table 9.1). In any acid–base disorder, the pH of the blood depends on the severity of the primary disturbance and the amount of compensation that has occurred.
Metabolic acidosis and alkalosis are the results of decreases and increases, respectively, in the concentration of HCO3–. These could be caused by the production of ketone bodies during diabetic ketoacidosis or from the loss of HCO3– from a duodenal fistula. Respiratory acidosis is associated with an increased PCO2 whereas respiratory alkalosis occurs when the PCO2 is decreased. For example, an impairment of respiratory function can increase the PCO2 in the blood while hyperventilation would decrease it.
Compensation of acid–base disorders occurs by two major mechanisms: renal compensation and respiratory compensation. Renal compensation occurs when a respiratory disorder impairs lung function. The body attempts to adjust the pH of blood back to within its reference range by increasing the excretion of H+ by the kidneys. Respiratory compensation is necessary when there is a metabolic acid–base disorder and involves changes in the ventilation of the lungs. Renal compensation is a relatively slow mechanism while respiratory compensation is much quicker to take effect. An acid–base disorder is said to be fully compensated if the compensatory mechanism returns the pH of the blood back to its reference range. However, compensation is usually partial and the pH remains outside the reference range.
METABOLIC ACID–BASE DISORDERS
Metabolic acid–base disorders lead to an accumulation or a loss of H+ resulting in changes in the concentration of HCO3– in the blood. The direct loss or gain of HCO3–will also cause a metabolic acid–base disorder. Thus metabolic disorders are recognized by investigating the concentration of HCO3– in the blood. Respiratory compensation occurs quickly, often within hours, and patients will show some change in blood PCO2 because of hypo-or hyperventilation.
Metabolic acidosis may arise from an increase in the amount of H+ formed or a decrease in the concentration of HCO3–. Diabetic ketoacidosis , lactic acidosis, poisoning with, for example, salicylate, methanol, ethylene glycol or the condition known as inherited organic acidosis all increase the production of H+. In contrast, a decreased excretion of H+ as in renal tubular acidosis, acute and chronic renal failure , the use of inhibitors of carbonic anhydrase or a deficiency of mineralocorticoid, such as aldosterone (Figure 9.8) all increase blood H+ content. Acid ingestion, as in acid poisoning, the excessive intake of amino acids by infusion, the direct loss of HCO3– by diarrhea or a pancreatic fistula can all reduce the concentration of HCO3– in the blood.
The clinical effects of metabolic acidosis include hyperventilation, where the increased H+ concentration acts as a rapid and powerful stimulant of the respiratory center leading to a deep sighing breathing called Kussmaulrespiration. The patients may also present with neuromuscular irritability thatcan cause cardiac arrhythmias. Cardiac arrest is more likely in the presence of hyperkalemia. Eventually metabolic acidosis can depress the activities of the central nervous system and this can progress to coma and even death. Patients with metabolic acidosis are managed by treating the underlying cause and this usually resolves the acid–base disorder. In severe cases, the patients may be administered HCO3– intravenously to correct the acidosis.
Metabolic alkalosis may occur as a consequence of gastrointestinal loss of H+ following vomiting and gastric aspiration or from excessive renal loss of H+ in Conn’s and Cushing’s syndromes . Some clinical treatments, such as the use of carbenoxolone, an anti-inflammatory drug used to treat ulcers, and thiazide diuretic drugs that reduce blood pressure by promoting the secretion of urine and by K+ depletion, can also result in this condition. Finally, the administration of alkali, including alkali ingestion, and inappropriate treatment for acidosis can also cause metabolic alkalosis.
The clinical effects of metabolic alkalosis include hypoventilation that is a consequence of the low H+ concentration. It is often accompanied by mental confusion and eventually coma. Patients may also suffer from paresthesia. Other effects of metabolic acidosis include tetany and muscle cramps that arise due to a decrease in the concentration of unbound Ca2+ in the plasma arising from the alkalosis. Metabolic alkalosis is usually managed by treating its underlying cause.
RESPIRATORY ACID–BASE DISORDERS
In respiratory acid–base disorders, the primary disturbance is caused by a change in the partial pressure of arterial CO2. Respiratory disorders are related to a defect in the rate of ventilation of lungs or the exchange of gases across the alveolar membrane. The changes in PCO2 alter the concentrations of carbonic acid in the blood, which, in turn, dissociates to HCO3– and H+.
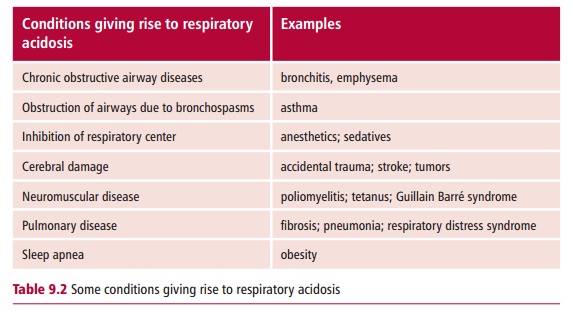
Some causes of respiratory acidosis are shown in Table 9.2. In general, obstruction of the airways by disease, or inhibition of the respiratory center in the brain by disease, trauma or drugs can cause respiratory acidosis.
Respiratory acidosis may be acute or chronic. Acute conditions occur within minutes or hours. It is usually the low PO2 (hypoxemia) that is more dangerous than the highPCO2 (hypercapnia). Further, renal compensation is slow, taking two or three days to become effective, so respiratory acidosis is usually uncompensated. Alveolar hypoventilation is usually the most common reason for acute respiratory acidosis. Hypoventilation increases the arterial PCO2and so the concentration of H+also rises quickly. The high PCO2andassociated low PO2 can cause coma and eventually death if untreated. Causes of acute respiratory acidosis include choking, bronchopneumonia and acute exacerbation of asthma.
Chronic respiratory acidosis is, again, usually due to a decline in alveolar ventilation. However, this is normally a well established condition and subject to maximum renal compensation. Long-standing conditions responsible for chronic respiratory disorders include chronic bronchitis and emphysema.
The high PCO2 is believed to be responsible for the clinical features of respiratory acidosis, such as peripheral vasodilatation and headaches. The acidosis can cause central nervous system depression leading to a coma. The treatment of respiratory acidosis is to improve alveolar ventilation, lowering the PCO2 and increasing the PO2. In chronic respiratory acidosis, it is usually not possible to treat the underlying cause and treatment is aimed at maximizing alveolar ventilation by using physiotherapy or bronchodilators.
Respiratory alkalosis is less common than respiratory acidosis. However, it is often an acute condition due to hyperventilation. Often renal compensation does not occur.
The clinical effects of respiratory alkalosis include confusion, headaches, dizziness and coma. Respiratory alkalosis may be caused by hypoxia, increased respiration or pulmonary disease. Hypoxia is associated with high altitudes, severe anemia and pulmonary disease. Increased respiration may result from the use of respiratory stimulants, such as salicylates, from primary hyperventilation syndrome, artificial hyperventilation, and pulmonary diseases, such as pulmonary edema and embolisms. The treatment of respiratory alkalosis is aimed at removing its underlying cause as this usually resolves the acid–base disturbance.
MIXED ACID–BASE DISORDERS
Sometimes patients may present with more than one acid–base disorder and this is known as a mixed acid–base disorder. These may present as (i) severe acidemia, that is a low blood pH, (ii) with a normal or near normal pH or (iii) with alkalemia, that is, a high blood pH. Whatever the underlying cause, all mixed acid–base disorders are associated with abnormal levels of blood PCO2 and HCO3–.
For example, a patient with chronic bronchitis may also have renal failure. Both these disorders increase the concentration of H+ in the blood. Chronic bronchitis leads to respiratory acidosis while the renal failure causes metabolic acidosis. This patient will therefore present with a mixed acid–base disorder with a high blood PCO2 and H+concentration but a low concentration of HCO3–. In some cases, however, the two disorders in a mixed acid–base disorder can be antagonistic, that is, have opposing effects on the concentration of H+ in blood. In this case the blood H+ concentration may be near normal although the PCO2 and HCO3– concentration will both be abnormal. For example, a patient with salicylate poisoning may have a metabolic acidosis together with a respiratory alkalosis. Patients may also present with metabolic and respiratory alkaloses. This could occur in someone with congestive cardiac failure who is on diuretic therapy. The former will cause a respiratory alkalosis and the latter a metabolic alkalosis. Such individuals will usually have a high blood pH and increased HCO3– but the PCO2 will be decreased.
Related Topics