Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Mycobacteria
Tuberculosis
TUBERCULOSIS
Tuberculosis is a systemic infection manifested only by evidence of an immune response in most exposed individuals. In some infected persons, the disease either progresses or, more commonly, reactivates after an asymptomatic period (years). The most common reactivation form is a chronic pneumonia with fever, cough, bloody sputum, and weight loss. Spread outside of the lung also occurs and is particularly devastating when it reaches the central nervous system. The natural history follows a course of chronic wasting to death aptly called “consumption” in the past.
EPIDEMIOLOGY
A recognized disease of antiquity, tuberculosis first reached epidemic proportions in the western world during the major periods of urbanization in the 18th and 19th centuries. Mortality reached 200 to 700 per 100,000 population each year, accounting for 20 to 30% of all deaths in urban centers and winning tuberculosis the appellation of the “white plague.” Morbidity was many times higher. The disease has had major sociologic compo-nents, flourishing with ignorance, poverty, overcrowding, and poor hygiene, particularly during the social disruptions of war and economic depression. Under these conditions, the poor are the major victims, but all sectors of society are at risk. Chopin, Paganini, Rousseau, Goethe, Chekhov, Thoreau, Keats, Elizabeth Barrett Browning, and the Brontës, to name but a few, were all lost to tuberculosis in their intellectual prime. With knowledge of the cause and transmission of the disease and the development of effective antimicro-bial agents, tuberculosis was increasingly brought under control in developed countries. Unfortunately, mortality and morbidity remain at 19th-century levels in many developing countries despite extensive national and international control programs.
The great majority of tuberculous infections are contracted by inhalation of droplet nuclei carrying the causative organism. Humans may also be infected through the gastrointestinal tract following the ingestion of milk from tuberculous cows (now uncommon due to pasteur-ization) or, rarely, through abraded skin. It has been estimated that a single cough can gener-ate as many as 3000 infected droplet nuclei and that less than 10 bacilli may initiate a pul-monary infection in a susceptible individual. The likelihood of acquiring infection thus relates to the numbers of organisms in the sputum of an open case of the disease, the frequency and efficiency of the coughs, the closeness of contact, and the adequacy of ventilation in the con-tact area. Epidemiologic data indicate that large doses or prolonged exposure to smaller in-fecting doses is usually needed to initiate infection in humans. In some closed environments, such as a submarine or a crowded nursing home, a single open case of pulmonary tuberculo-sis can infect the majority of nonimmune individuals sharing sleeping accommodations.
In the past, an animal variant (Mycobacterium bovis) was transmitted by drinking milk from infected herds. This disease has been largely eliminated by eradication pro-grams and milk pasteurization.
The decline in mortality and occurrence of the disease in the United States over the last century is shown in Figure 28 – 2. Between 1953 to 1985 the number of new tubercu-losis cases per annum fell from 84,304 to 22,201. By the mid-1980s, it was estimated that only 4 to 5% of American citizens, and less than 1% of American children, demonstrated positive tuberculin skin tests. However, the decline was not uniform throughout the American populace, and case rates among nonwhites and the urban poor remained signif-icantly higher than the national average. As the incidence of infection in the United States and other developed countries decreased, there was also a major shift in the age of tuber-culosis patients. Most were over 50 years of age and represented cases in which an old primary lesion, quiescent for decades, became reactivated. The grandfather who has de-veloped “chronic bronchitis” is a classic source of infection to children.
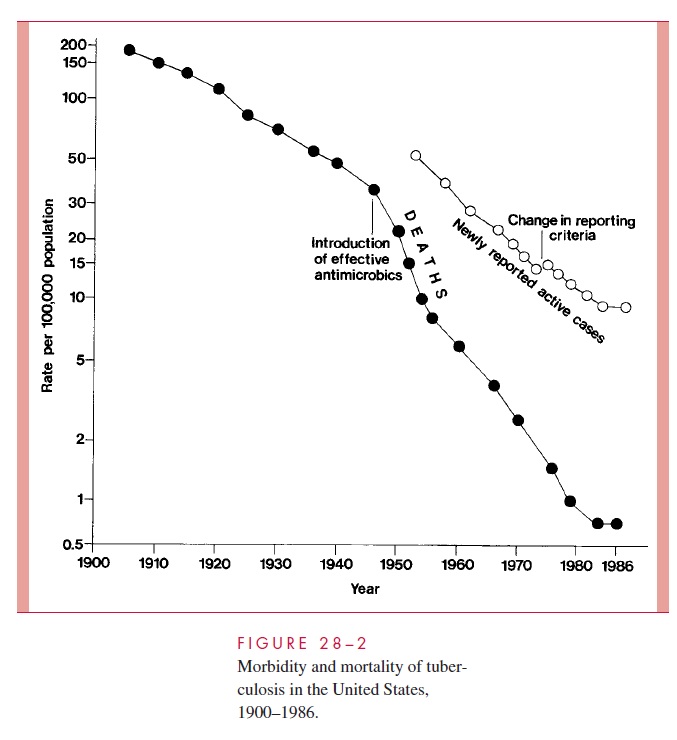
In 1985, the steady decline in reports of new tuberculosis cases and deaths in the United States ceased, and, in the ensuing 7 years, new cases increased by nearly 20%.
This change has been attributed to a significant decrease in the funding for tuberculosis control programs; spread of multiresistant strains of M. tuberculosis; increased immigra-tion from tuberculosis-endemic areas of the world; social and economic changes that con-tributed to a burgeoning of incarcerated intravenous drug users and homeless populations; and, finally, to the AIDS epidemic. It is estimated that patients with latent tuberculosis in-crease their risk of reactivation disease by factors of 200 to 300 with the development of a human immunodeficiency virus (HIV) coinfection. The per annum reactivation rate of such individuals is estimated at 8%.
Accompanying the increase in reactivation tuberculosis among high-risk US popula-tions was an increase in the transmission of M. tuberculosis. Annual tuberculin skin con-versions among intravenous drug abusers and the number of cases of tuberculosis among children under 5 years of age increased significantly between 1987 and 1990. Single-source epidemics involve school children and a teacher with unrecognized pulmonary tu-berculosis, homeless shelters, nursing homes, and medical personnel exposed to patients with unrecognized tuberculosis. Since 1992, a reinvigorated public health effort in the United States has, again, led to a declining number of individuals with active tuberculo-sis, reaching a new low of 18,361 in 1998. This represents a rate of 6.8/100,000 popula-tion, still well short of the nation’s interim goal of 3.5/100,000 for the year 2000 and 1/100,000 for the year 2010.
Globally, the situation is more ominous. It is estimated that one third of the world’s population is infected with M. tuberculosis; 30 million people have active disease, an additional 8 million develop new disease yearly, and 2 to 3 million die annually of this “captain of death.” As a result, tuberculosis is the leading cause of death from an infec-tious disease worldwide. It is thought responsible for 6% of all deaths and 26% of avoidable adult deaths. Particularly concerning for the future control of tuberculosis worldwide is the marked susceptibility of patients with AIDS and the growing resis-tance of M. tuberculosis to the currently available antimicrobic agents. Because 40% of all new cases of tuberculosis in the United States are among foreign-born individuals, the elimination of this disease in the United States will be impossible without a sub-stantial reduction in the global burden of tuberculosis.
PATHOGENESIS
Primary Infection
Primary tuberculosis is the response to the initial infection in an individual not previously infected and sensitized to tuberculoprotein. Inhaled droplet nuclei containing small num-bers of tubercle bacilli are deposited in the peripheral respiratory alveoli, most frequently those of the well-ventilated middle and lower lobes. Here they are engulfed by nonspecif-ically activated alveolar macrophages. The ability of these cells to destroy ingested or-ganisms depends significantly on their inherent microbicidal capacity. If the alveolar macrophages are unable to destroy ingested mycobacteria, they continue to multiply until the macrophage bursts. The released organisms are subsequently ingested by inactivated blood macrophages that, together with T cells, are attracted to the lung by chemotactic factors.
The ingested mycobacteria continue to multiply intracellularly without damage to their host cell. Some of the bacterial-laden macrophages are transported through lym-phatic channels to the hilar lymph nodes draining the infected site. From there, they may disseminate through blood and lymphatic systems to a number of tissues, including the liver, spleen, kidney, bone, brain, meninges, and apices or other parts of the lung. The in-flammatory reaction in the seeded tissues is usually minor, and the signs and symptoms of infection are absent. However, the primary site of infection and some enlarged hilar lymph nodes can often be detected radiologically. In infants and immunocompromised adults, hematogenous dissemination of organisms may occasionally produce a life-threat-ening meningitis.
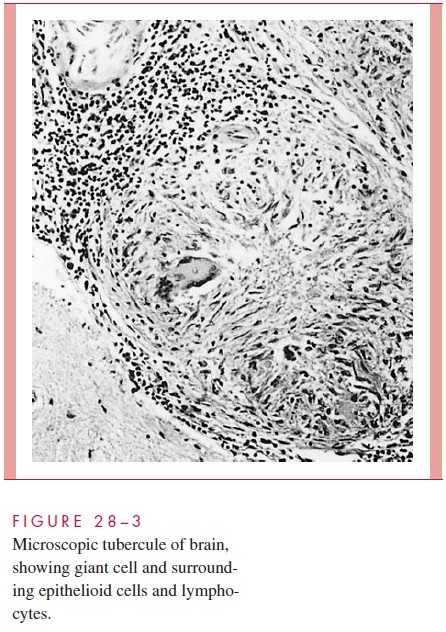
Morphologically, the resulting tubercle is a microscopic granuloma comprised of some multinucleated giant cells formed by the fusion of several macrophages (Langhans cells), many epithelioid cells (activated macrophages), and a surrounding collar of lym-phocytes (Fig 28 – 3) and fibroblasts. When many bacteria are present and there is a high degree of hypersensitivity, enzymes, reactive oxygen intermediates, and reactive nitrogen intermediates are released by dying macrophages and lead to necrosis of the center of the granuloma, which is termed caseous because of the cheesy, semisolid character of the gross lesion.
Primary infections are usually handled well by the host. Bacterial multiplication ceases. Most microscopic lesions heal by fibrosis, and the organisms in them slowly die. In others, especially those in well-oxygenated tissues such as the subapical areas of the lung, renal cortex and vertebral bodies, the tubercle bacilli remain viable for long periods and serve as a potential source of reactivation many months or years later if host defenses weaken.
Reactivation (Adult) Tuberculosis
Reactivation usually occurs in body areas of relatively high oxygen tension and low lym-phatic drainage, most often in the apex of the lung. The lesions show spreading, coalescing tubercles with numerous tubercle bacilli, and large areas of caseous necrosis. Necrosis often involves the wall of a small bronchus from which the necrotic material is discharged, result-ing in a pulmonary cavity and bronchial spread. Frequently, small blood vessels are also eroded. The chronic fever and weight loss may be mediated in part by macrophage-derived tumor necrosis factor.
Virulence Mechanisms
The basis for M. tuberculosis virulence is largely unknown. It produces no exotoxins, and both the intact cell and cellular components are remarkably innocuous to humans and ex-perimental animals not previously sensitized to tuberculin. Cell wall components such as LAM have been implicated in binding to alveolar macrophages, utilizing surface fi-bronectin, mannose, or complement receptors (CR1, CR3). Once inside, multiple factors contribute to survival and continued multiplication. A number of genes have been identi-fied that are linked to virulence by enhancing survival in the macrophage or by influenc-ing the physical and chemical conditions (low pH, high lactic acid, high CO2) present in developing lesions, but their function remains unknown. Mycolic acids, sulfolipids, LAM, and proteins have been shown to disrupt phagosome – lysosome interactions and interfere with oxidative killing. LAM has also been shown to modulate cytokine produc-tion and downregulate other aspects of T-cell function including antigen presentation.
IMMUNITY
Humans generally have a rather high innate immunity to development of disease. This was tragically illustrated in the Lübeck disaster of 1926 where infants were administered M. tuberculosis instead of an intended vaccine strain. Despite the large dose, only 76 of249 died and most of the others developed only minor lesions. Approximately 10% of im-munocompetent persons infected with M. tuberculosis will develop active disease any time in their life. There is epidemiologic and historic evidence for differences in the im-munity in certain population groups and between identical and nonidentical twins.
DTH to tuberculoprotein and CMI to M. tuberculosis develop 2 to 6 weeks after primary infection. The subsequent course of the infection depends on the balance between these two defensive mechanisms. DTH, through the mediation of natural killer cells, destroys the inac-tivated macrophages as well as the surrounding tissues, releasing still viable mycobacteria into an area of necrosis unsuitable for bacterial multiplication. CMI develops when compe-tent T lymphocytes recognize mycobacterial antigen complexes on the surface of M. tubercu-losis – containing macrophages. In the presence of macrophage-produced interleukin-1, theactivated lymphocytes respond to the presented antigens with the elaboration of several cytokines. Some of these proteins attract circulating monocytes. Others, including interferon-and possibly tumor necrosis factor- , activate local tissue macrophages and the recruited monocytes to enhanced destruction of ingested mycobacteria, resulting in a slowing or discontinuation of intracellular bacterial growth. Nitrous oxide or other reactive nitrogen in-termediates probably mediate the destruction of the mycobacteria. Another cytokine, inter-leukin-2, induces clonal expansion of the activated lymphocytes, thus amplifying the host’s immunologic response. Still others stimulate accumulation of fibroblasts and deposition of collagen, which help wall off the area of infection and prevent further dissemination.
Acquired immunity is cell mediated but incomplete. Both helper – inducer (CD4+) and cytotoxic (CD8+) T lymphocytes are involved. Two to three weeks after infection, macrophages are activated at the site of infection by a network of pro- and anti-inflammatory cytokines and chemokines from antigen-stimulated CD4+ T lymphocytes, macrophages, and dendritic cells. This interaction between M. tuberculosis and the host is what eventually limits its multiplication and spread. Cytotoxic T cells release bacilli from inac-tivated phagocytic cells and allow them to be ingested and handled by the activated macrophages. The concomitant DTH to tuberculoprotein plays an important part in im-munity to reinfection by mobilizing immune cells and macrophages to the site of deposi-tion of tubercle bacilli. In the past, it was believed that reinfection from external sources was extremely rare, but it is now clear that loss of hypersensitivity and CMI can occur over time and that reinfection can develop into clinical tuberculosis.
The role of DTH in immunity of established tuberculosis is complex, because high degrees of sensitivity can precipitate caseous necrosis and lead to spread of the disease. The importance of CMI and hypersensitivity in modulating the course of tuberculosis is, perhaps, most dramatically illustrated in patients with AIDS. Those with minimal impair-ment of cellular immune responses develop typical tubercles containing relatively few bacilli. Those with advanced impairment demonstrate abundant acid-fast bacilli without epithelioid cell accumulation or associated tissue necrosis.
Related Topics