Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Theory of Capillary Electrophoresis
Theory of Capillary Electrophoresis
In capillary electrophoresis the sample is injected into a buffered
solution retained within a capillary tube.
When an electric field is applied
to the capillary tube, the sample’s
components migrate as the result of two types of mobility: elec- trophoretic mobility and electroosmotic mobility. Electrophoretic mobility
is the solute’s response to the applied
electric field. As described earlier, cations move toward the negatively
charged cathode, anions move toward the positively
charged anode, and neutral species,
which do not respond to the electric
field, re- main stationary. The other contribution to a solute’s migration is electroosmotic
flow, which occurs
when the buffer
solution moves through
the capillary in re-
sponse to the applied electric field. Under normal
conditions the buffer
solution moves toward the cathode, sweeping most solutes, even anions,
toward the nega- tively charged cathode.
Electrophoretic Mobility
The velocity with which a solute moves
in response to the
applied electric field
is called its electrophoretic velocity, vep; it is defined
as
vep = μepE ……………..12.35
where μep is the solute’s
electrophoretic mobility, and E is the magnitude of the ap- plied electric field. A solute’s electrophoretic mobility is defined
as

where q is the solute’s charge,
μ is the buffer solvent’s
viscosity, and r is
the solute’s radius. Using equations 12.35 and 12.36, we can make several
important conclu- sions about
a solute’s electrophoretic velocity. Electrophoretic mobility, and, there- fore, electrophoretic velocity, is largest
for more highly
charged solutes and solutes
of smaller size. Since q is positive for cations and negative for anions, these species
migrate in opposite
directions. Neutral species, for which q is 0, have an elec- trophoretic velocity
of 0.
Electroosmotic Mobility
When an electric field is applied
to a capillary filled with an
aqueous buffer, we expect the
buffer’s ions to migrate in response to their elec- trophoretic mobility. Because the solvent, H2O, is neutral, we might reasonably ex- pect it to remain stationary. What is observed
under normal conditions, however, is that the buffer solution
moves toward the cathode. This phenomenon is called the electroosmotic flow.
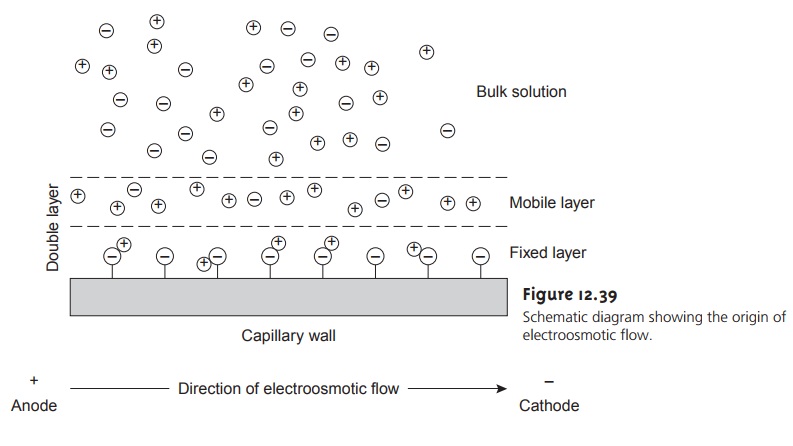
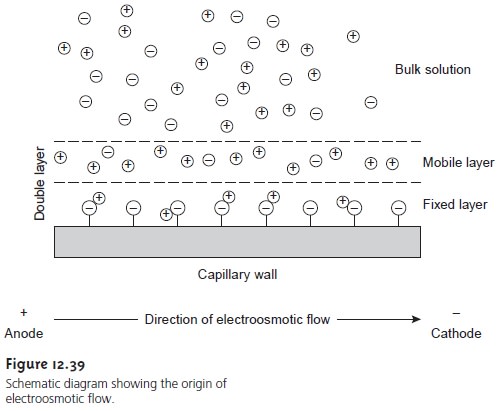
Electroosmosis occurs because
the walls of the capillary
tubing are electrically charged. The surface of a silica
capillary contains large
numbers of silanol
groups (Si–OH). At pH levels greater
than approximately 2 or 3, the silanol
groups ionize to form negatively charged
silanate ions (Si–O–). Cations from the buffer are at- tracted to the silanate
ions. As shown
in Figure 12.39,
some of these
cations bind tightly to the silanate
ions, forming an inner, or fixed, layer. Other cations
are more loosely bound,
forming an outer,
or mobile, layer.
Together these two layers are called the double layer. Cations in the outer layer migrate
toward the cathode.
Be- cause these cations
are solvated, the
solution is also
pulled along, producing the electroosmotic flow.
Electroosmotic flow
velocity, veof, is a function of the magnitude of the applied electric field and the
buffer solution’s electroosmotic mobility, μeof.
veof = μeofE ……………..12.37
Electroosmotic mobility is defined as

where ε is the buffer
solution’s dielectric constant, ξ is the
zeta potential, and
ÎĽ is the buffer
solution’s viscosity.
Examining equations 12.37
and 12.38 shows
that the zeta potential plays an
important role in determining the
electroosmotic flow velocity. Two factors deter- mine the zeta potential and, therefore, the electroosmotic velocity. First, the zeta potential is directly proportional to the charge on the capillary walls, with a greater
density of silanate ions corresponding to a larger
zeta potential. Below
a pH of 2, for example, there are few silanate ions;
thus, the zeta potential and electroosmotic flow velocity are 0. As the pH level is increased, both the zeta potential and the electroos- motic flow velocity increase. Second, the zeta potential is proportional to the thick- ness of the double layer. Increasing the buffer solution’s ionic strength provides
a higher concentration of cations, decreasing the thickness of the double
layer.
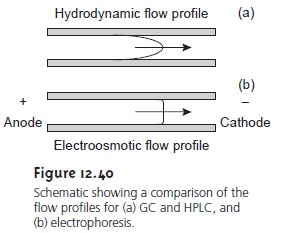
The electroosmotic flow profile is very different from that for
a phase moving under forced
pressure. Figure 12.40
compares the flow profile for electroosmosis with that for hydrodynamic pressure. The uniform, flat profile
for
electroosmosis helps to minimize band broadening in capillary elec- trophoresis, thus improving separation efficiency.
Total Mobility
A solute’s net, or total velocity, v, is the sum of its elec- trophoretic velocity and the electroosmotic flow velocity; thus,
vtot = vep + veof
ÎĽtot = ÎĽep + ÎĽeof
Under normal conditions the following relationships hold
(vtot)cations > veof
(vtot)anions < veof
(vtot)neutrals = veof
Thus, cations elute first in an order corresponding to their electrophoretic mobili- ties, with small,
highly charged cations
eluting before larger
cations of lower
charge. Neutral species elute
as a single band, with
an elution rate
corresponding to the electroosmotic flow velocity. Finally,
anions are the last components to elute, with smaller, highly charged anions
having the longest
elution time.
Migration Time
A solute’s total velocity is given by
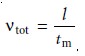
where l is the distance the solute travels
between its point
of injection and the detec- tor, and tm is the migration time. Since
vtot
= ÎĽtotE = (ÎĽep + ÎĽeof)E
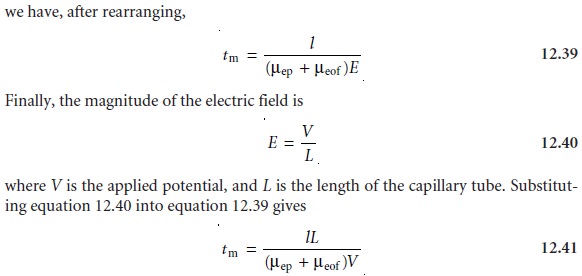
Examining equation 12.41
shows that we can decrease
a solute’s migration time (and thus the total analysis
time) by applying
a higher voltage
or by using a shorter capillary tube. Increasing the electroosmotic flow also shortens
the analysis time, but, as we will see shortly,
at the expense of resolution.
Efficiency
The efficiency of
capillary electrophoresis is characterized by the num- ber of theoretical plates,
N, just as it is in GC or HPLC. In capillary electrophoresis, the number of theoretic
plates is determined by

where D is the
solute’s diffusion coefficient. From equation 12.42
it is easy to see that the efficiency of a capillary electrophoretic separation increases with higher
voltages. Again, increasing the electroosmotic flow
velocity improves efficiency, but at the expense
of resolution. Two additional observations deserve comment.
First, solutes with
larger electrophoretic mobilities (in the same
direction as the electroosmotic flow) have greater efficiencies;
thus, smaller, more highly charged solutes
are not only
the first solutes
to elute, but
do so with greater efficiency. Sec- ond, efficiency in capillary electrophoresis is independent of the capillary’s length. Typical theoretical plate counts are approximately
100,000–200,000 for capillary electrophoresis.
Selectivity
In chromatography, selectivity is defined as the ratio
of the capacity factors for two solutes
(equation 12.11). In capillary electrophoresis, the analogous
expression for selectivity is
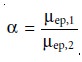
where ÎĽep,1 and ÎĽep,2 are the electrophoretic mobilities for solutes
1 and 2, respec- tively, chosen
such that α >= 1. Selectivity often
can be improved by adjusting the pH of the
buffer solution. For
example, NH4+ is a weak acid
with a pK of
9.24. At a pH
of 9.24 the concentrations of NH4+ and NH are equal. Decreasing the pH below9.24
increases its electrophoretic mobility because a greater fraction
of the solute is present as the cation
NH4+. On the other hand,
raising the pH above 9.24 increases
the proportion of the neutral
NH3, decreasing its
electrophoretic mobility.
Resolution
The resolution between two
solutes is
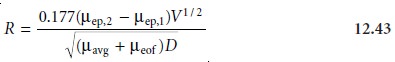
where ÎĽavg is the average
electrophoretic mobility for the two solutes. Examining equation 12.43 shows that increasing the applied voltage
and decreasing the elec-
troosmotic flow velocity
improves resolution. The latter effect is particularly impor- tant because increasing electroosmotic flow improves
analysis time and efficiency
while decreasing resolution.
Related Topics