Chapter: Plant Anatomy:An Applied Approach: The leaf
The epidermis - Leaf structure
The epidermis
![]()
![]() Leaf surfaces must be mechanically adapted to meet environmental stress-es, but translucent, to allow photosynthetically active radiation to pass through them to reach the pigment chlorophyll in cells beneath.
Leaf surfaces must be mechanically adapted to meet environmental stress-es, but translucent, to allow photosynthetically active radiation to pass through them to reach the pigment chlorophyll in cells beneath.
Cuticle and cuticular sculpturing
In all but the wettest environments, leaf surfaces must also be capable of helping to reduce water loss. This is helped in many species by the presence of a transparent outer layer, the cuticle, which retards water loss. This tends to be thinnest in species not normally subject to water stress, and thickest in those that are. Angiosperms with submerged leaves may have an exceed-ingly thin cuticle, or it may be absent. The cuticle may also give added me-chanical strength. It helps resist abrasion by blown sand particles, or in the case of some conifers, blown ice crystals. The main component of cuticle is cutin, which may permeate the walls of epidermal cells, or just the outer walls. It is most developed in species confined to extremely arid habitats. Low availability of water to the plant can be induced by saline soils, so plants growing on these often show adaptations similar to those from dry habitats.
The cuticle and the outer part of the wall of the epidermis that it covers and grades into is patterned or sculptured in many plants. If the sculpturing is of low relief it will not show strongly in sections and may be faint in surface view. The strong sculpturing in Aloe, for example, may often be obscured by the granular appearance of the interface between cuticle and epidermis (Fig. 6.4).
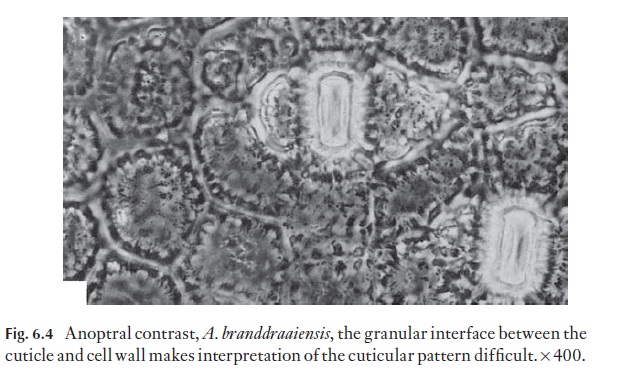
Although many patterns can be seen with the light microscope, either on intact cells or with detached cuticles or surface replicas, the scanning elec-tron microscope is important in surface studies.
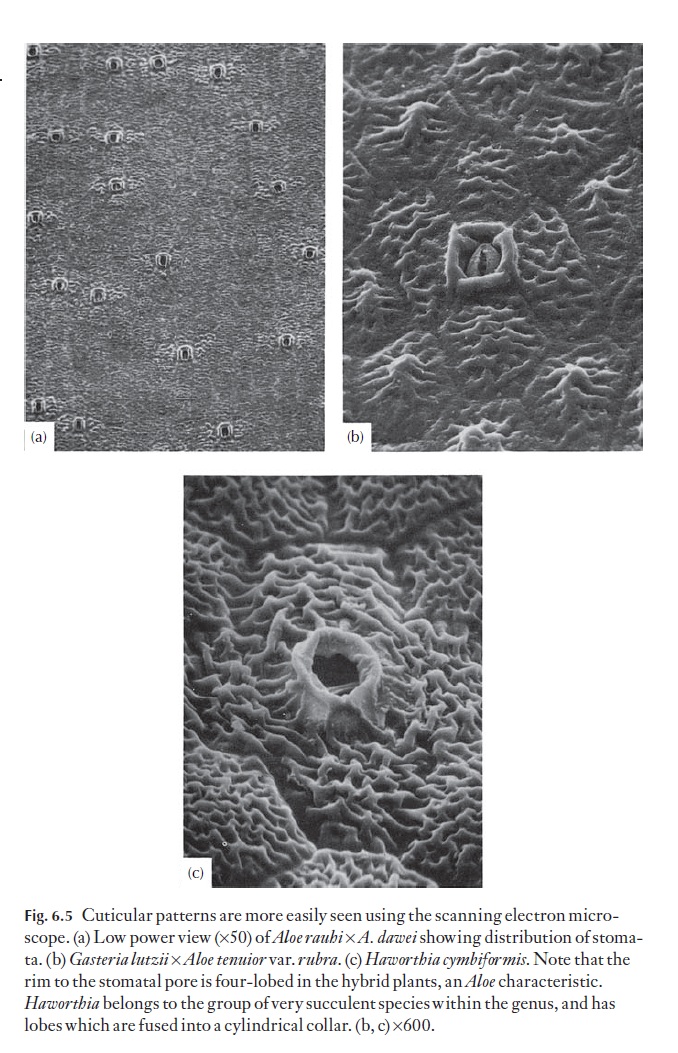
In aloes and haworthias, the range of cuticular outer cell wall patterns is such that individual species or groups of species can often be identified by their particular pattern. Striations are quite common, as are micropapillae. Some patterns are shown in Fig. 6.5.
Sometimes the cuticle and its markings are masked by a covering of wax. Most people are familiar with the waxy ‘bloom’ on apples and plums, and have noticed that some leaves have a dull sheen on them, for example, as in the cabbage (Brassica) or in Agave. Few may realize that many other plants also have a waxy crystalline covering, because this may be very thin and eas-ily removed. Other chemicals such as flavenoids are sometimes involved. Surface wax may be smooth, or may show varying degrees of roughness. It may function in helping reduce water loss, but it has reflecting and other properties.
Many plants with xeromorphic characteristics have a waxy covering, which retards cuticular water loss.
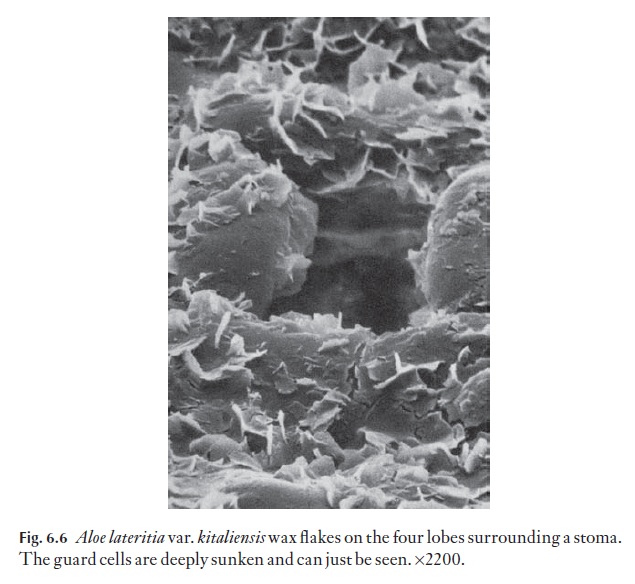
Wax takes on many crystalline forms, and may also be present as a melted-down layer. Wax in a few species may go through a daily cycle in which wax crystals of one form melt, and recrystallize into another form, but this is rare. Many xeromorphic monocotyledons have large numbers of their stomata plugged by wax. The scanning electron micrograph in Fig. 6.6 shows some wax flakes in Aloe lateritia var.kitaliensis. Wax embellish-ment is often associated with sunken stomata.
The appearance of leaf surface sculpturing can be complex. However, by using a straightforward procedure, the sculpturing can be broken down into four elements, for description.
Primary sculpturing defines the overall arrangement of cells, generally visible at low magnification.
Secondary sculpturing defines: (i) the orientation and shapes of the cells and describes the number of anticlinal walls, whether they are straight, or if not, the degree of sinuosity; how distinct they are, as ridges or chan-nels, for example (e.g. cells six-sided, as long as to twice as long as wide, with straight sunken anticlinal walls; Fig. 6.5b) (ii) details of the outer (periclinal) wall (e.g. flat, concave, convex with a pronounced central papilla; in Fig. 6.5c they are low-domed); (iii) the position, type and frequency of stomata.
Tertiary sculpturing is the finer detailed sculpturing found on the outer periclinal wall, superimposed on the primary sculpturing. It may be absent, and the surface is then described as smooth. It includes micropapillae, and defines their size and distribution (e.g. fine, covering the whole cell surface), striae, their thickness, distribution and orienta-tion (e.g. coarse, longitudinally oriented striae, along the long axis of the cell, or striae forming a reticulum as in Fig. 6.5c).
Quaternary sculpturing includes epicuticular secretions, for example wax, farinose material (some Primulaspecies). This may be present as smooth layers, upright flakes with random (e.g. Fig. 6.6) or defined orientation; coarse or fine amorphous particles, filaments or tubes, for example. See the references in the further reading.
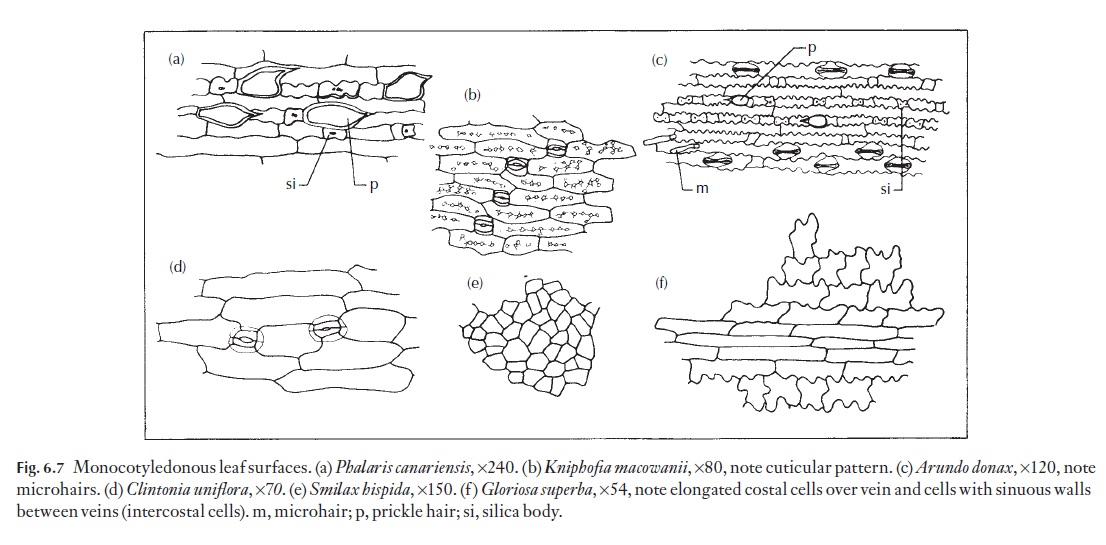
Epidermal cells vary a great deal from species to species, particularly as seen in surface view. Many monocotyledons, and in particular those with strap-shaped or axially elongated leaves, have elongated cells that are arranged in well-defined longitudinal files. These cells may be 4–6 or more sided; their anticlinal walls may be straight, curved or sinuous. Sometimes the outlines of these walls are more sinuous near the cell outer wall than near the inner wall. Figure 6.7 shows a range of named examples of cell forms.
The epidermal cells of leaves of grasses fall into two distinct classes, described in the literature on grass anatomy as ‘long’ and ‘short’ cells. These two size classes should not be confused with variations in cellular dimensions that are to be seen over veins (costal cells) and between veins
The true ‘short’ cells are frequently suberized, or they may contain silica bodies. Even small fragments of leaf from a member of the Poaceae can often be identified to the family level, based upon the epi-dermal cell characters.
The majority of dicotyledons and many monocotyledons without axially elongated leaves (strap-shaped), for example Smilax, Gloriosa, tend to have epidermal cells of irregular shape and size. They have straight, curved or sinuous anticlinal walls. Because dicotyledon leaves lack a basal meristem, but grow in area by regions of cell division, their epidermal cells are rarely arranged in clear rows. Figure 6.8 shows a range of cell types from named plants.
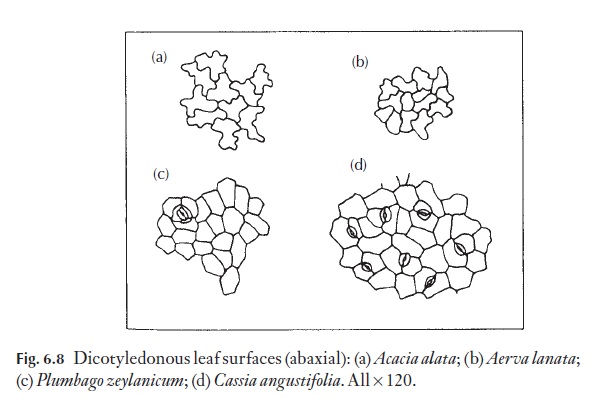
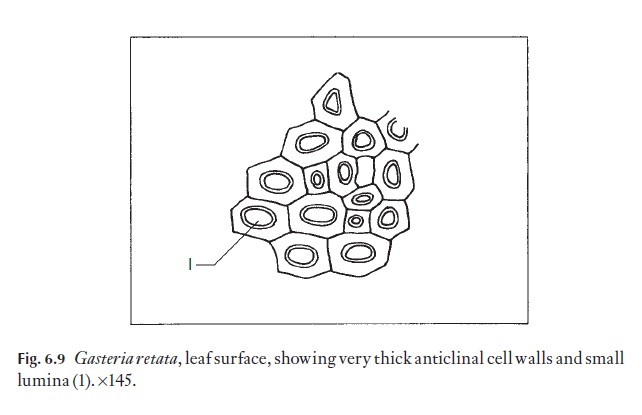
Anticlinal walls of the epidermal cells of both monocotyledons and dicotyledons can be very thin and hardly visible from the surface, or they may range through degrees of thickness to very thick, so that the lumen of the cells appears from the surface to be very reduced (Fig. 6.9). In dicoty-ledons, as in monocotyledons, the costal cells frequently differ from those of intercostal regions; they tend to be elongated in the direction of the veins.
Sometimes the cells of the upper and lower surfaces of leaves may be sim-ilar in size and structure, but more often they are not alike. In monocotyle-dons with true dorsiventral leaves, adaxial and abaxial epidermal cells may differ markedly in size, and the adaxial epidermis, may contain bulliform (‘motor’) cells that are considerably larger than the normal epidermal cells. The dissimilarity may be in cell size and wall thickness, or merely the absence of stomata from one surface.
Cells at the margins and the tip of the leaf are often narrower than the rest, and have thicker walls. Some marginal cells may develop into unicellu-lar or multicellular prickles.
Measurements of epidermal cells have been made to try and distinguish between closely related species. If enough careful measurements are made and a statistical analysis carried out, significant differences may be detect-ed. Unfortunately, this seemingly valuable method is limited in usefulness by the natural variation in size within different specimens of the same species, or even among cells from different leaves on the same plant. Sun and shade leaves, for example, can differ in this respect.
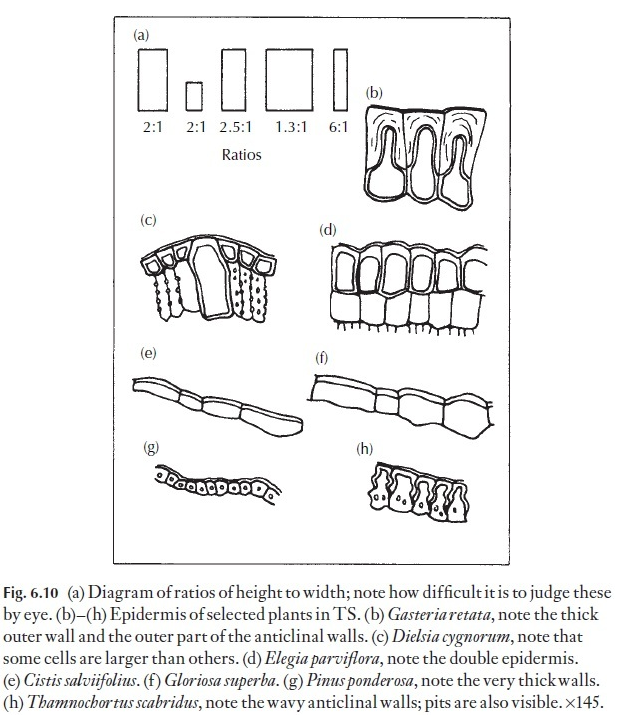
Even if absolute size differences may seem to be unreliable in many instances in distinguishing between species, the proportion of length to width of epidermal cells can often give useful data for comparison. This length: width ratio can be fairly constant in a species, even if the cell size varies phenotypically. The importance of selecting leaves for comparison from comparable positions on the various plants under study cannot be overstressed. Normally, one would select mature, vigorous leaves. The eye can play tricks, and it is easy to be misled about length: breadth ratios unless they are actually measured – look at the diagrams in Fig. 6.10. Here, dia-grams showing various height: width ratios are illustrated, together with diagrams of the epidermis of named plants in transverse section.
Many leaves capable of rolling up in dry, unfavourable conditions, and reopening again under conditions when there is no water stress, have spe-cial, thin-walled water-containing cells that enable them to make these movements. These are the bulliform or motor cells. Examples may be found in many grasses, for example, marram grass, Ammophila arenaria, and many members of the bamboosoid grasses. The shape, size and disposition of such cells can be used as an aid to classification and identification. Cells with similar properties are present at the pulvinus and at the attachment regions of the leaflets to the rachis in many plants whose leaves fold at night.
Stomata
The main control of water movement is provided by stomata. These consist of a pair of guard cells (often kidney-shaped) with a pore between them. The size of the pore is regulated by changes in shape of the guard cells, and is under active control, unless the plant is so dehydrated that it wilts. As hydraulic pressure is altered, the cells deform in a regulated way, aided by specialized, uneven wall thickening. When plants wilt, the stomata may open, and this can lead to damage.
Stomata may be present on both surfaces (amphistomatic), or only on the upper (hypertomatic) or only on the lower (hypostomatic) surface. They may occur at the same general level as surrounding epidermal cells, or they may be sunken below the general surface of the leaf as in cycads. In broad-leaved plants, stomata tend to have a scattered distribution, whilst innarrow leaved species, stomata are generally arranged in rows which are parallel to the longitudinal axis of the leaf blade. In some xerophytic plants (e.g. Nerium oleander) stomata are sunken beneath the abaxial leaf surface within stomatal crypts. In some angiosperms with aerial leaves, the distri-bution may vary from species to species, depending to some extent on the degree of xeromorphy or mesomorphy. They are characteristically absent from submerged aquatic leaves, but are present on the upper surface of float-ing leaves, for example Nymphaea and Victoria.
![]()
![]() As mentioned, stomata may be superficial, that is, with the guard cells level with the surface of the leaf, or sunken, with a small outer chamber above the guard cells. Although many xerophytes have sunken stomata, and the majority of mesophytes superficial stomata, this is not invariably the rule. There may be particular adaptive advantages in each arrangement under certain circumstances; it may not be clear why some apparently ‘unadapted’ species survive while others around them are modified to a greater or lesser degree, but the timing of leaf emergence and their fall, or physiological adaptations, for example, may also play a part.
As mentioned, stomata may be superficial, that is, with the guard cells level with the surface of the leaf, or sunken, with a small outer chamber above the guard cells. Although many xerophytes have sunken stomata, and the majority of mesophytes superficial stomata, this is not invariably the rule. There may be particular adaptive advantages in each arrangement under certain circumstances; it may not be clear why some apparently ‘unadapted’ species survive while others around them are modified to a greater or lesser degree, but the timing of leaf emergence and their fall, or physiological adaptations, for example, may also play a part.
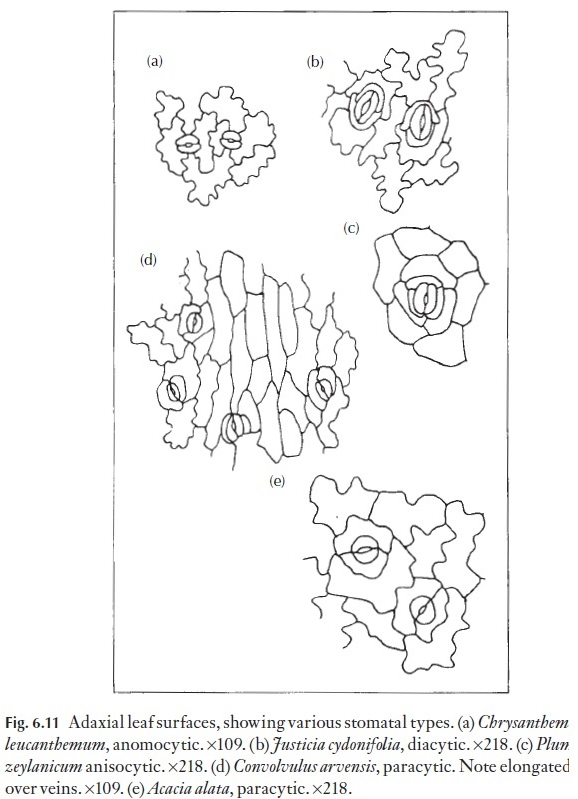
Of great interest to the taxonomist, or to the person wishing to identify a small leaf fragment, is the arrangement of subsidiary cells where these are present. Some of the various common types are illustrated in Fig. 6.11. Those stomata that lack subsidiary cells are called anomocytic, where the cells surrounding each stoma are not recognizably different or distinct from the remaining cells in the mature epidermis. Such stomata occur in the Ranunculaceae, for example. Stomata with two subsidiary cells, one at either pole, are called diacytic; Justicia and Dianthus species have such sto-mata. Stomata with two subsidiary cells, one on either flank (i.e. laterally), are termed paracytic; these occur in for example,Juncus, Sorghum, Carex and Convolvulus species. The paracytic type also includes species with a number of subsidiary cells in a parallel arrangement on either flank. Tetracytic stomata, with four subsidiary cells, can be seen readily in Trades-cantia; here one cell occurs on either flank and one at either pole. If there arethree cells of unequal size surrounding the guard cell pair the stoma is called anisocytic, as in Plumbago and members of the Brassicaceae. Cyclocytic sto-mata have a ring of subsidiary cells of approximately equal size and in which the individual cells are not very wide, whereas in the actinocytic type the subsidiary cells radiate strongly. Naturally enough, there can be intermedi-ate forms that cannot easily be classified. Aberrant forms are also frequent, for example, two paracytic stomata may share one of the subsidiary cells.
Although occasional species exist which have several types of stomata on a leaf, most have one type only. This means that by noting the type of stoma present, the identity of a plant can be narrowed down. Of course, many families share the more common paracytic and tetracytic types, so the combination of all characters available must be seen to fit with reference material before identification can be made. There are other stomatal types, and indeed the ferns provide some interesting forms, the polocytic with the guard cell pair towards one end of a single subsidiary cell and the mesocytic type, where the guard cell pair is in the centre of a subsidiary cell are two such examples.
It is all too easy to think that some forms of arrangement of subsidiary cells must be primitive, and some more advanced. By speculating, phyloge-netic sequences can be postulated, and interrelationships suggested. One great danger in doing this arises because a mature stomatal type may be formed by more than one developmental sequence in different groups of plants. Perhaps we need two systems of naming stomatal types, the first taking into account the mature form and used only for identification, and
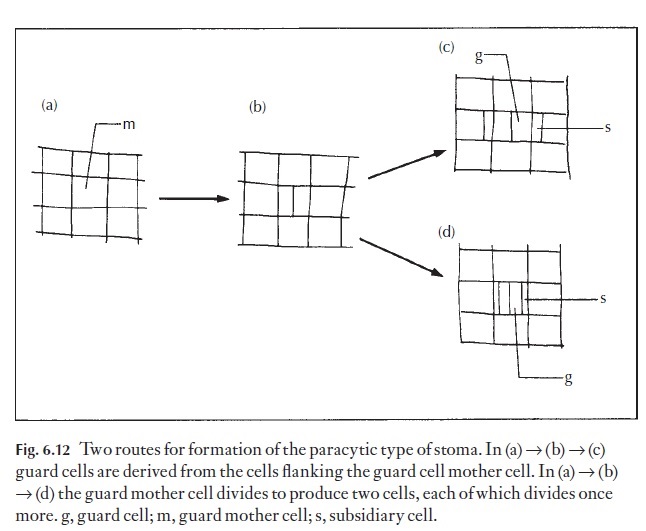
the second derived from a study of the ontogeny of the stomata and used by the phylogeneticist or taxonomist. Figure 6.12 shows two possible ways by which paracytic stomata may arise. In the first route the guard cell mother cell (meristemoid) divides first to produce two cells, then each of the flank-ing cells divides to form one subsidiary cell. The second pathway involves the division only of the guard cell mother cell. Two flanking cells may divide to form one subsidiary cell each, either before or after division of this cell.
Sometimes mature stomata may appear at first sight to have no subsidiary cells. A study of the early stages of development could show that cells sur-rounding the guard cell mother cell divide in a particular way that differs from that which normally occurs among the other epidermal cells. Many aloes appear to have four subsidiary cells, whereas up to eight cells may sur-round the stomata. These subsidiary cells have oblique anticlinal walls. Most other cells in areas not adjacent to stomata have transverse walls. The oblique walls are the product of additional divisions in cells next to the guard cell mother cell.
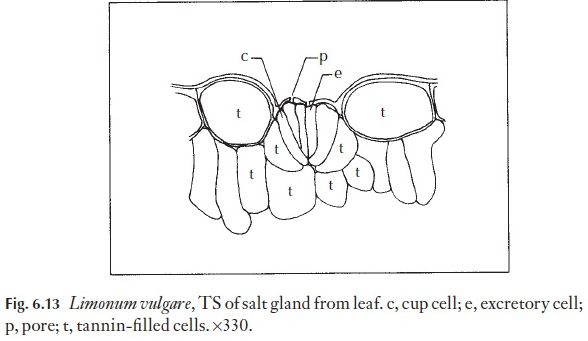
Sometimes stomata are specialized to exude droplets of liquid water. They may simply be ‘giant’ stomata, larger than the others on the leaf, as in some members of Anacardiaceae. They might be specialized, and elevated at the end of a small mound situated at the termination of a small veinlet. Structures through which droplets of water may exude but which have non- functional guard cells are called hydathodes. Salt glands are a type of hyda-thode modified for the exudation of salt water. They are often surrounded by an encrustation of salt. Examples of hydathodes may be found in saxi-frages and salt glands in Limonium (Fig. 6.13).
Crystals and silica bodies can occur in the epidermis but for convenience will be described in the ‘mesophyll’ section.
Trichomes
Hairs and papillae (and scales) are collectively called trichomes. Taxono-mists use their occurrence and cellular structure extensively as an aid to identification, because there is such a wide range of form. When a plant possesses hairs or papillae, they are usually of a type or types charac-teristic of that species. It should be noted that various samples of a plant of a given species may range from being glabrous (hairless) to very hirsute (hairy). This means that the numbers and density of hairs can be a poor character to use taxonomically, except, perhaps, in defining subspecies or varieties, if there are other, linked characters to support those divisions. Also, although hairs are so diverse in form, there are very few types that can be used even in the diagnosis of a family, that is, are of taxonomic significance.
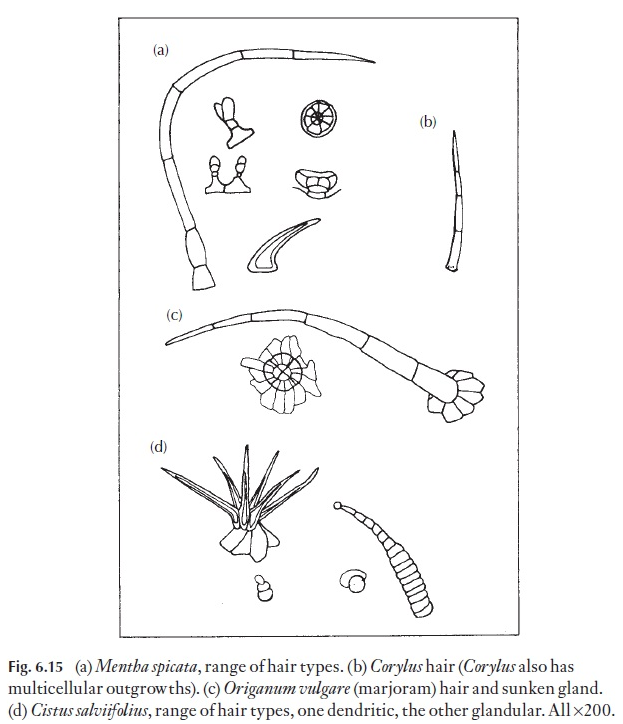
The greatest value of hairs is in identification, that is, they have high di-agnostic value. They are constant in a species when present, or show a con-stant range of form. Consequently, small fragments of leaf with hairs can often be matched with known material. If you look at the descriptions of powdered drugs of leaf origin in the European Pharmacopoea or your national pharmacopoea you will see the hairs carefully defined. Examina-tion of hair types can help in quality control, for example of dried herbs, like mint, where cheaper substitutes may have been added (Fig. 6.15).
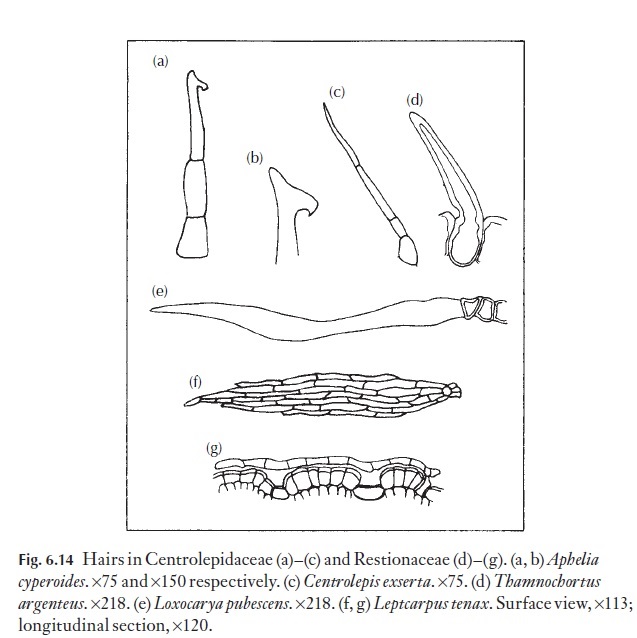
In some families, individual species can be defined on the form of their hairs alone. Among these are Restionaceae and Centrolepidaceae which show good examples of simple, unbranched hairs (Fig. 6.14). The relative sizes of the basal cell and the cells of the free portion vary from species to species. The curious ‘boathook’ end of the cell in Aphelia cyperoides is diag-nostic. Gaimardia has complex, branching filamentous hairs.
In the Restionaceae, Leptocarpus from Australia, New Zealand, Malaysia and South America, flattened, shield-shaped stem hairs occur. These hairs are multicellular, diamond-shaped plates, held closely to the surface of the stem on short, sunken stalks as shown in Fig. 6.14. Until recently it was thought that Leptocarpus also occurred in South Africa, but the hair type and other internal histological differences show that the South African plants really belong to a distinct genus, which at that stage in the investiga-tion, had not been named. A close relative to Leptocarpus in Australia is Meeboldina, which have diamond-form hairs, and two large, thin-walledtranslucent central cells, together with a border of thick-walled cells withrecurved micropapillae which effectively zip adjacent hairs together so that they will strip off in a sheet if you try to pull one away.
Some plants have hairs on both upper and lower surfaces but in many cases they are confined to the lower surface. Examples of leaves with hairs on both surfaces are to be found among the silver-leaved composites. The air in the hairs masks the chlorophyll in the leaf, giving a highly refractive, silvery or white appearance.
Hairs are divided into two major categories, the glandular and non-glandular (or covering) hairs. Glandular hairs (Fig. 6.16) include the sting-ing hairs of plants like the nettle, Urtica. Less familiar are the irritant hairs from the pods of Mucuna, the ‘cowitch’ (Fabaceae), from the West Indies (Fig. 6.16a).
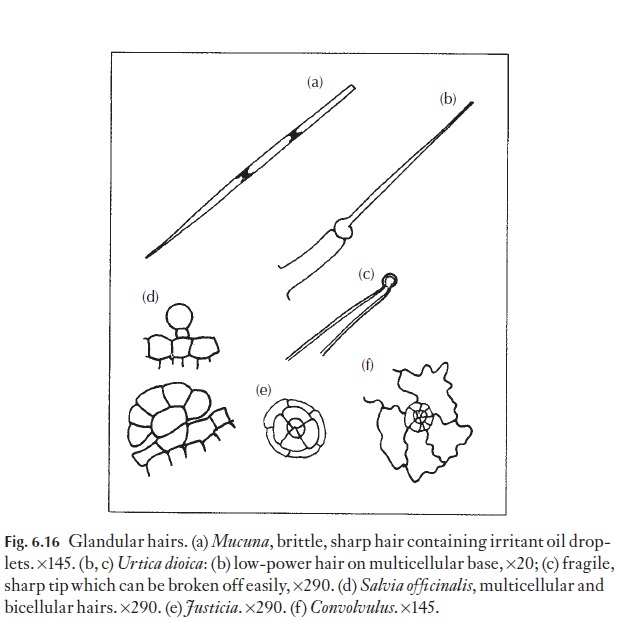
Simple glandular hairs are present on the leaves of plants that can trap and digest small insects and other small animals. Some of these are sticky, and some specialized to secrete digestive enzymes. Pinguiculaand Drosera are examples.
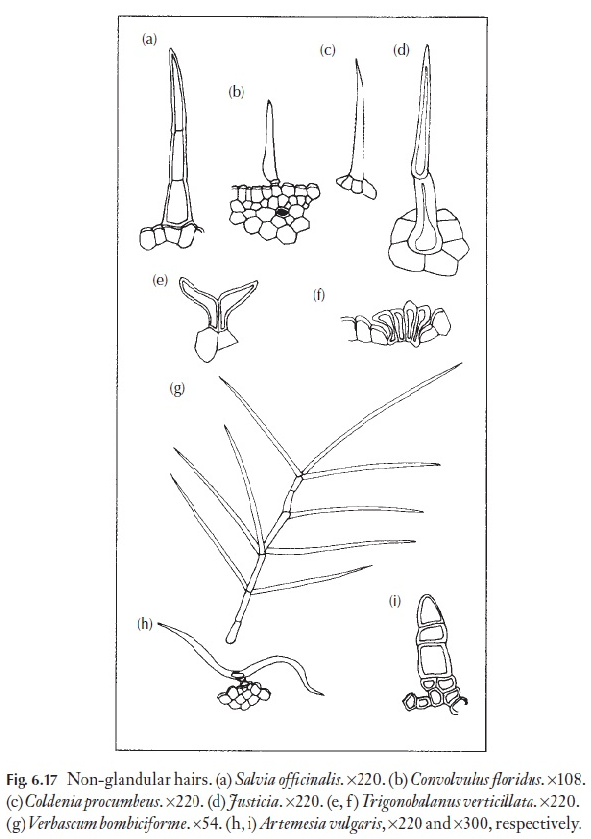
Some of the fragrant (or less pleasant) essential oils occur in glandular hairs or leaves. The non-glandular hairs are much more varied and diverse than the glandular. A range of types are illustrated in Fig. 6.17, and the plants on which they occur are identified in the caption. One or more of these plants or its relatives should grow near your home. The larger hairs will be visible with a hand lens.
As mentioned earlier, usually the hair type is only one of many characters that may be used in identification. However, some families are easily recog-nized by their hairs, for example the T-shaped hairs of Malpighiaceae (Fig. 6.17h). Rhododendrons have been classified on the basis of leaf hairs, as an aid to the identification of species. Here, not only form, but also hair colour is used in the keys.
Microhairs are very short, two-celled hairs that are present on the leaves of some grasses, mostly from the tropics. Prickle hairs, which are usually prominent on margins and veins of grasses, are normally unicellular. They have thick walls that can be silicified. This is why it is easy to cut your hands on some grasses, and why cattle are selective in their grazing.
A number of hairs are of commercial value. These are not leaf hairs, but usually come from the fruit or seed, for example cotton (Gossypium) and kapok (Bombax).
The function of hairs is generally thought to be related to the water rela-tions of a leaf. A densely hairy surface would tend to restrict the rate of flow of drying air. In xerophytes, hairs frequently have a suberin band in the wall towards their base. This prevents water leakage from the leaf through the cell wall of the hairs (apoplastic movement). Hairs, of course, increase con-siderably the potential surface area for evaporation. The converse require-ment is true of hairs on epiphytes like Tillandsia (Bromeliaceae). In these plants, which are not rooted in the ground, the hairs are able to absorb water from rain or mist. They lack suberin bands. It is easy to test for ‘waterproof’
hairs. A piece of hairy leaf surface is cut carefully from a leaf and floated on a Petri dish containing a solution of calcofluor white for an hour, then re-moved and viewed in UV light (while wearing protective goggles). If there are suberin bands, the hairs will not fluoresce. If bands are absent, the hairs will fluoresce brightly. However, other hairs seem to have primarily an anti-herbivore function as in the grasses described above.
Scales have a wide base, are usually one to a few cell layers thick, and lack any vascular tissue. Their form, size and position can be used diagnosti-cally. They are frequent on fern fronds.
Bulliform cells
Many leaves that are capable of rolling up in dry, unfavourable conditions, and reopening again under conditions when there is no water stress, have special, thin-walled water-containing cells that enable them to make these movements. These are the bulliform or motor cells. Examples may be found in grasses, for example marram grass, Ammophila arenaria, and many mem-bers of the bamboosoid grasses. The shape, size and disposition of such cells can be used as an aid to classification and identification. (Fig. 6.18).
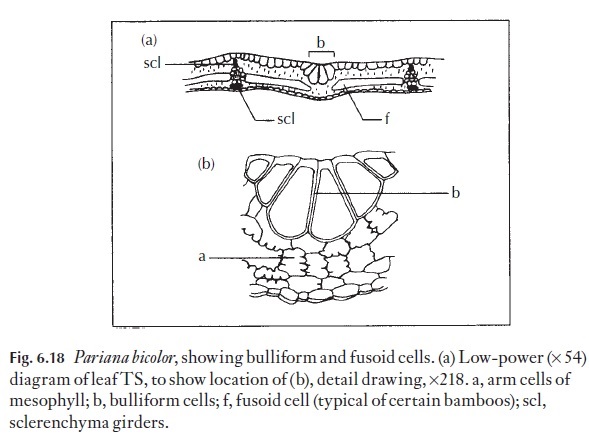
Cells with similar properties are present at the pulvinus and at the attach-ment regions of the leaflets to the rachis in many plants whose leaves fold at night.
Hypodermal layers
Xerophytic floras typically may have a large number of species in which a hypodermal layer is well-developed. As the name suggests, these special- ![]()
![]() ized cells are present to the inner side of and next to the epidermal cells. The cells within hypodermal layers typically contain few chloroplasts and are often thick-walled. Hypodermal cells are derived from cortical cells, not the epidermis. Some species with xerophytic anatomy such as Nerium olean-der have a multiple layered hypodermis.
ized cells are present to the inner side of and next to the epidermal cells. The cells within hypodermal layers typically contain few chloroplasts and are often thick-walled. Hypodermal cells are derived from cortical cells, not the epidermis. Some species with xerophytic anatomy such as Nerium olean-der have a multiple layered hypodermis.
Related Topics