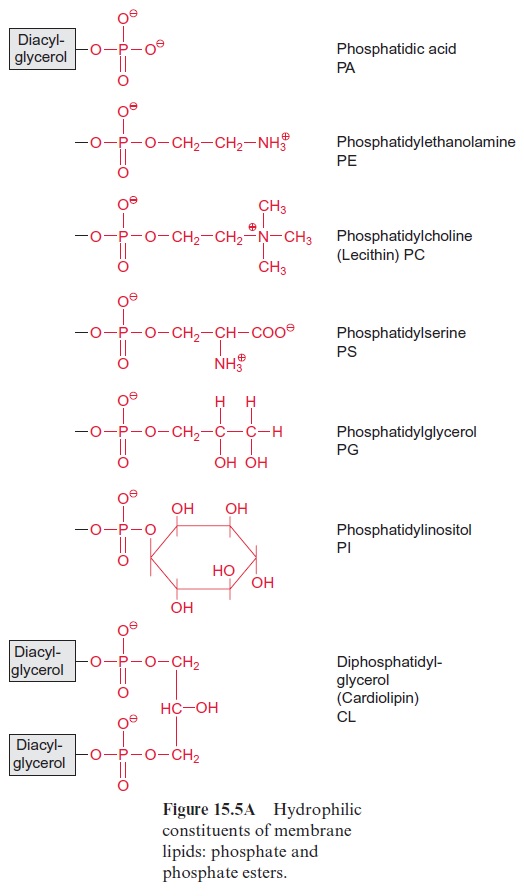
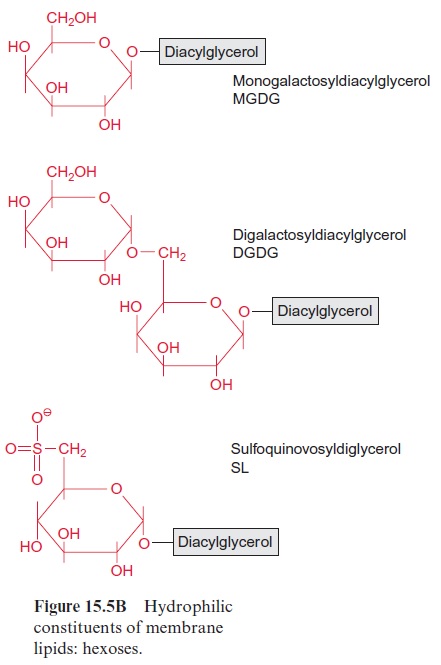
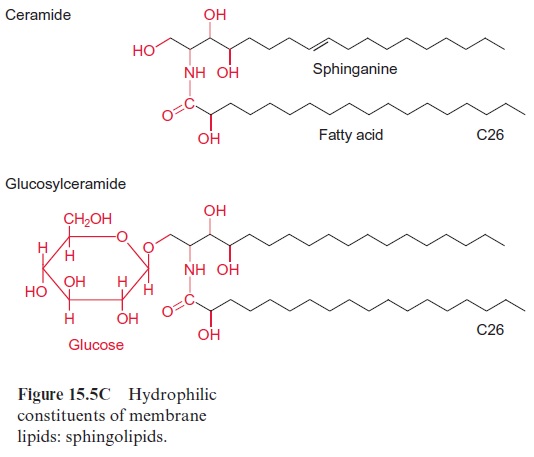
Chapter: Plant Biochemistry: Lipids are membrane constituents and function as carbon stores
The de novo synthesis of fatty acids takes place in the plastids
The de novo synthesis of fatty acids takes place in the plastids
The carbon fixed by CO2 assimilation in the chloroplasts is the precursor not only for the synthesis of carbohydrates and amino acids. Whereas the production of carbohydrates and amino acids by the mesophyll cells is primarily destined for export to other parts of the plants, the synthesis of fatty acids occurs only for the cell’s own requirements, except in seeds and fruits. Plants are not capable of long-distance fatty acid transport. Since fatty acids are present as constituents of membrane lipids in every cell, each cell must contain the enzymes for the synthesis of membrane lipids and thus also for the synthesis of fatty acids.
In plants the de novo synthesis of fatty acids always occurs in the plas-tids: in the chloroplasts of green cells and the leucoplasts and chromoplasts of non-green cells. Although in plant cells enzymes of fatty acid synthesis are also found in the membrane of the ER, these enzymes appear to be involved only in the modification of fatty acids, which have been synthesized earlier in the plastids. These modifications include a chain elongation of fatty acids, as catalyzed by elongases and the introduction of further double bonds by desaturases (Fig. 15.15B).
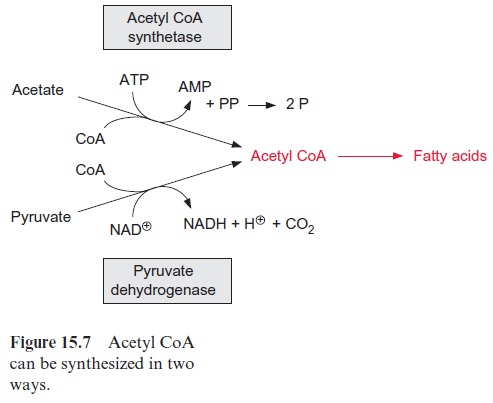
Acetyl CoA is a precursor for the synthesis of fatty acids
Acetyl CoA is provided in different ways. Like mitochondria (see Fig. 5.4), plastids contain a pyruvate dehydrogenase complex, by which pyruvate is oxidized to acetyl CoA, accompanied by the reduction of NAD+ (Fig. 15.7). In chloroplasts, however, depending on the developmental state of the cells, the activity of pyruvate dehydrogenase is often low. On the other hand, chloroplasts contain a high activity of acetyl CoA synthetase, which can convert acetate upon consumption of ATP to acetyl CoA. In many plants, acetate is often a major precursor for the formation of acetyl CoA in the chloroplasts and leucoplasts. Thus, when chloroplasts are supplied with radioactively labeled acetate, the radioactivity is very rapidly incor-porated into fatty acids. Our knowledge about the origin of the acetate is still fragmentary. One possibility is that it is formed in the mitochondria by hydrolysis of acetyl CoA, which derived from the oxidation of pyruvate by the mitochondrial pyruvate dehydrogenase complex.
In chloroplasts, photosynthesis provides the NADPH required for the synthesis of fatty acids. In leucoplasts, the NADPH required for fatty acid synthesis is provided by the oxidation of glucose 6-phosphate via the oxida-tive pentose phosphate pathway (Fig. 6.21). Glucose 6-phosphate is trans-ported by a glucose phosphate-phosphate translocator to the plastids (see Fig. 13.5).
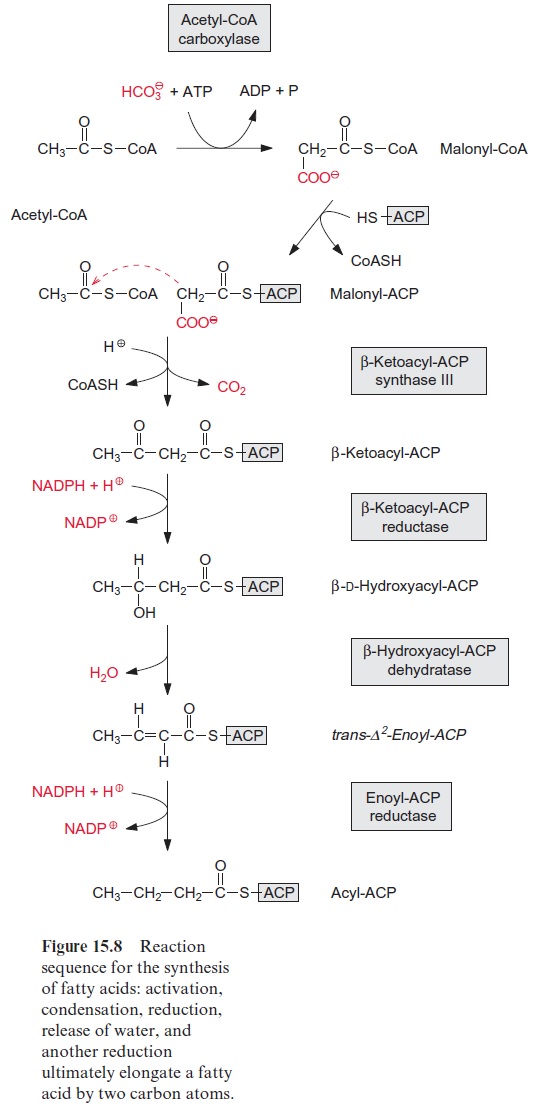
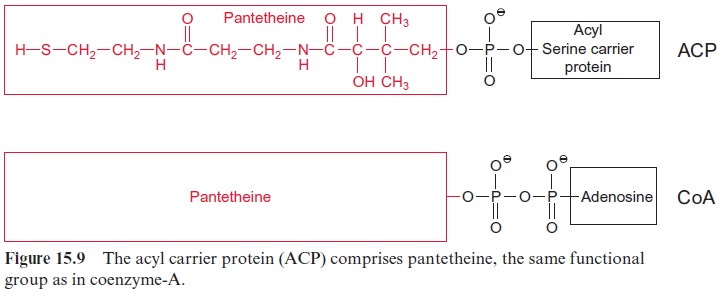
Fatty acid synthesis starts with the carboxylation of acetyl CoA to malo nyl CoA by acetyl CoA carboxylase, with the consumption of ATP (Fig. 15.8). In a subsequent reaction, CoA is exchanged by acyl carrier protein (ACP) (Fig. 15.9). ACP comprises a serine residue to which a pantetheine is linked via a phosphate group. The pantetheine is also a functional consti tuent of CoA. Both ACP and CoA are covalently bound to a protein. The enzyme β-ketoacyl-ACP synthase III (KAS III) catalyzes the condensation of acetyl CoA with malonyl-ACP. The reaction is irreversible due to the libe ration of CO2. The function of the enzymes KAS I and KAS II will be discussed later (Fig. 15.14). The acetoacetate thus formed remains bound as a thioester to ACP and is reduced by NADPH to β-D-hydroxyacyl-ACP. Following the release of water, the carbon-carbon double bond formed is reduced by NADPH to produce acyl ACP. The product is a fatty acid that has been elongated by two carbon atoms (Fig. 15.8).
Acetyl CoA carboxylase is the first enzyme of fatty acid synthesis
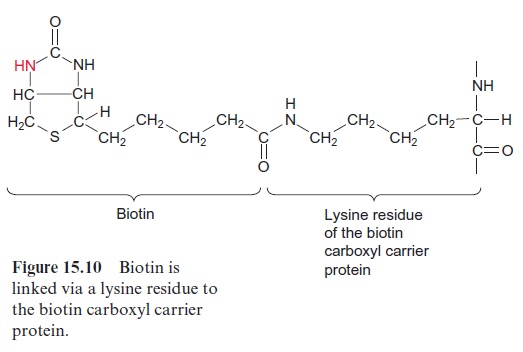
The carboxylation of acetyl CoA involves biotin which acts as a carrier for “activated CO2” (Fig. 15.10). Biotin is covalently linked with its carboxyl group to the e-amino group of a lysine residue of the biotin carboxyl carrier protein, and its -NH-group can form a carbamate with HCO3- (Fig. 15.11). This reaction is driven by the hydrolysis of ATP. Therefore the acetyl CoA carboxylation requires two steps:
1. Biotin is carboxylated at the expense of ATP by biotin carboxylase.
2. Bicarbonate is transferred to acetyl CoA by carboxyl transferase.
All three proteins—the biotin carboxyl carrier protein, biotin carboxy-lase, and carboxyl transferase—form a single multienzyme complex. Since the biotin is attached to the carrier protein by a long flexible hydrocarbon chain, it reacts alternately with the carboxylase and carboxyl transferase in this multienzyme complex (Fig. 15.11).
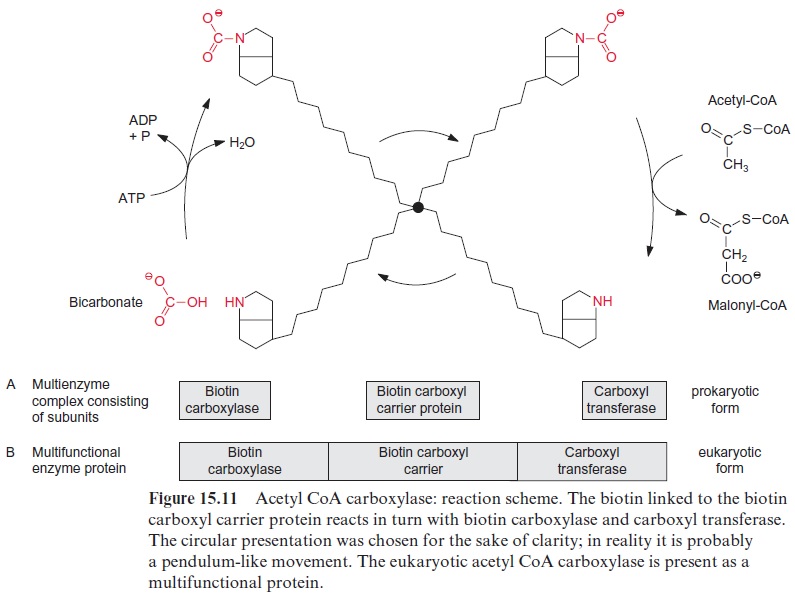
The acetyl CoA carboxylase multienzyme complex in the stroma of plas-tids consists of several subunits, resembling the acetyl CoA carboxylase in cyanobacteria and other bacteria, and is referred to as the prokaryotic form of the acetyl CoA carboxylase. Acetyl CoA carboxylase is also present out-side the plastids, probably in the cytosol. The malonyl CoA formed outside the plastids is used for chain elongation of fatty acids and is the precursor for the formation of flavonoids. The extra-plastidic acetyl CoA carboxylase, in contrast to the prokaryotic type, is a single large multi functional protein in which the biotin carboxyl carrier, the biotin carboxy-lase, and the carboxyl transferase are located on different sections of the same polypeptide chain (Fig. 15.11). Since this multifunctional protein also occurs in a very similar form in the cytosol of yeast and animals, it is referred to as the eukaryotic form. It should be emphasized, however, that the eukaryotic form as well as the prokaryotic form of acetyl CoA carboxy-lase are encoded in the nucleus. Possibly only one protein of the prokaryotic enzyme is encoded in the plastid genome.
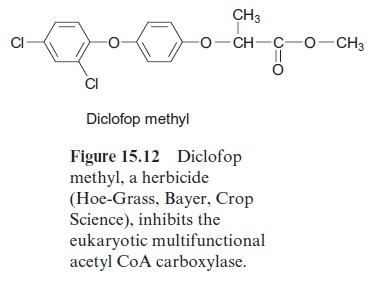
In Gramineae (grasses), including the various species of cereals, the prokaryotic form is not present. In these plants, the multifunctional eukaryotic acetyl CoA carboxylase is located in the cytosol as well as in the chloroplasts. The eukaryotic acetyl CoA carboxylase is inhibited by vari-ous arylphenoxypropionic acid derivatives, such as, for example, diclofop methyl (Fig. 15.12). Since eukaryotic acetyl CoA carboxylase in Gramineae is involved in the de novo fatty acid synthesis of the plastids, this inhibitor severely impairs lipid biosynthesis in this group of plants. Diclofop methyl (trade name Hoe-Grass, Bayer, Crop Science) and similar substances are therefore used asselective herbicides to control grass weeds.
Acetyl CoA carboxylase, the first enzyme of fatty acid synthesis, is an important regulatory enzyme and its reaction is regarded as a rate-limiting step in fatty acid synthesis. In chloroplasts, the enzyme is fully active only during illumination and is inhibited during darkness. This ensures that fatty acid synthesis proceeds mainly during the day, when photosynthesis provides the necessary NADPH. The mechanism of light regulation is simi-lar to the light activation of the enzymes of the Calvin cycle : The acetyl CoA carboxylase is reductively activated by thioredoxin and the activity is further enhanced by the increase of the pH and the Mg++ con-centration in the stroma.
Further steps of fatty acid synthesis are also catalyzed by a multienzyme complex
β-Ketoacyl ACP formed by the condensation of acetyl CoA and malonyl ACP (Fig. 15.8) is reduced by NADPH to -D-hydroxyacyl ACP, and after the release of water the carbon-carbon double bond of the resulting enoyl ACP is reduced again by NADPH to acyl ACP. This reaction sequence resembles the reversal of the formation of oxaloacetate from succinate in the citrate cycle (Fig. 5.3). Fatty acid synthesis is catalyzed by a multien-zyme complex. Figure 15.13 shows a schematic presentation of the inter-play of the various reactions. The ACP, comprising the acyl residue bound as a thioester, is located in the center of the complex. Thus the acyl residue is attached to a flexible chain, to be transferred from enzyme to enzyme during this reaction cycle.
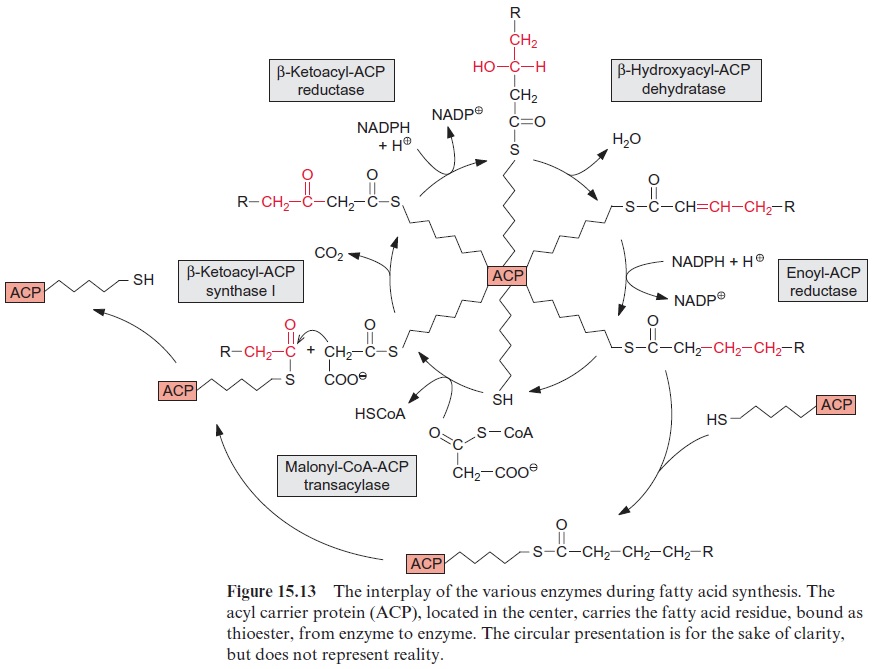
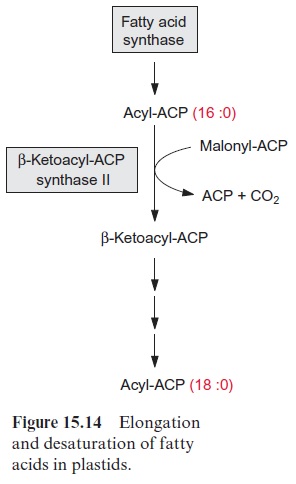
A fatty acid is elongated by transferring it to another ACP which is then condensed with malonyl ACP. The enzyme β-ketoacyl-ACP synthase I, catalyzing this reaction, enables the formation of fatty acids with a chain length of up to C-16. A further chain elongation to C-18 is catalyzed by β- ketoacyl-ACP synthase II (Fig. 15.14).
It should be mentioned that in animals and fungi the enzymes of fatty acid synthesis (Fig. 15.13) are present in one multifunctional protein, or two multifunctional proteins which form a complex (eukaryotic fatty acid synthase complex). Since the fatty acid synthase complex of the plastids, consisting of several proteins, is similar to those of many bacteria, it is called the prokaryotic fatty acid synthase complex.
The first double bond in a newly synthesized fatty acid is formed by a soluble desaturase
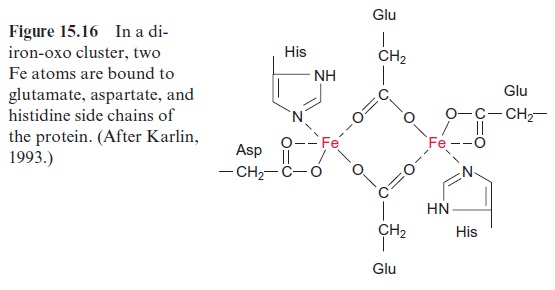
The synthesized stearoyl ACP (18:0) is desaturated to oleoyl ACP (18:1) in the plastid stroma (Fig. 15.15A). This reaction can be regarded as a monooxygenation , in which one O atom from an O2 molecule is reduced to water and the other is incorporated into the hydrocarbon chain of the fatty acid as hydroxyl group (Fig. 15.15B). A carbon-carbon double bond is formed by subsequent liberation of H2O (analogous with the β-hydroxyacyl ACP dehydratase reaction, Fig. 15.8), which, in contrast to fatty acid synthesis, has a cis-configuration. The monooxygenation requires two electrons, which are provided by NADPH via reduced ferredoxin. Monooxygenases are widespread in bacteria, plants, and animals. In most cases, O2 is activated by a special cytochrome, cytochrome P450. However, in the stearoyl ACP desaturase, the O2molecule reacts with a di-iron-oxo cluster
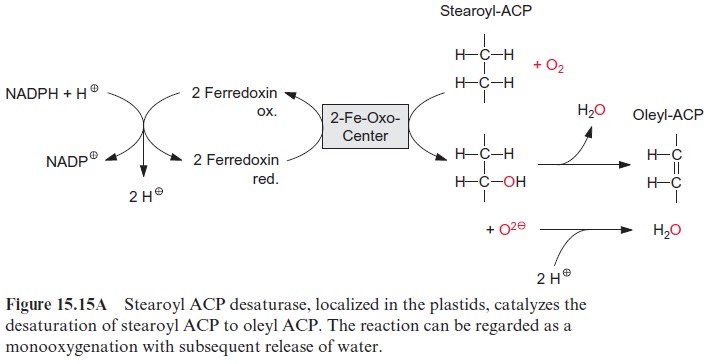
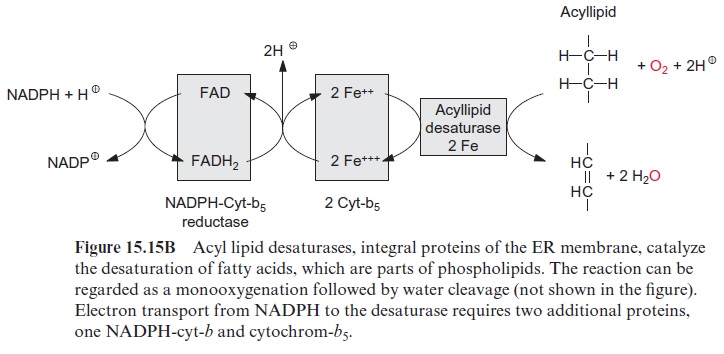
Fig. 15.16). In previous sections, we have discussed iron-sulfur clusters as redox carriers, in which the Fe atoms are bound to the protein via cysteine residues (Fig. 3.26). In the di-iron-oxo cluster of the desaturase, two iron atoms are bound to the enzyme via the carboxyl groups of glutamate and aspartate. The two Fe atoms alternate between oxidation state +IV,+ III and II. An O2 molecule is activated by the binding of the two Fe atoms.
Stearoyl ACP desaturase is a soluble protein that is localized in chloro-plasts and other plastids. The enzyme is so active that normally the newly formed stearoyl ACP is almost completely converted to oleyl ACP (18:1) (Fig. 15.17). This soluble desaturase is capable of introducing only one double bond into fatty acids. The introduction of further double bonds is catalyzed by other desaturases, which are integral membrane proteins of the ER and of the plastidal inner envelope membrane. These desaturases react only with fatty acids that are constituents of membrane lipids. For this reason, they are termed acyl lipid desaturases. These membrane-bound desaturases also require O2 and reduced ferredoxin, similar to the aforemen-tioned ACP desaturases, but have a different electron transport chain (Fig. 15.15B). The required reducing equivalents are transferred from NADPH via an FAD containing NADPH-cytochrome-b5-reductase to cytochrome-b5 and from there further to the actual desaturase, which contains two Fe atoms probably bound to histidine residues of the protein. In plastids ferre-doxin acts as a reductant. The acyl lipid desaturases belong to a large family of enzymes. Members of this family catalyze the introduction of hydroxyl groups (hydroxylases), epoxy groups (epoxygenases), conjugated double bonds (conjugases), and carbon triple bonds (acetylenases) into fatty acids of acyl lipids.
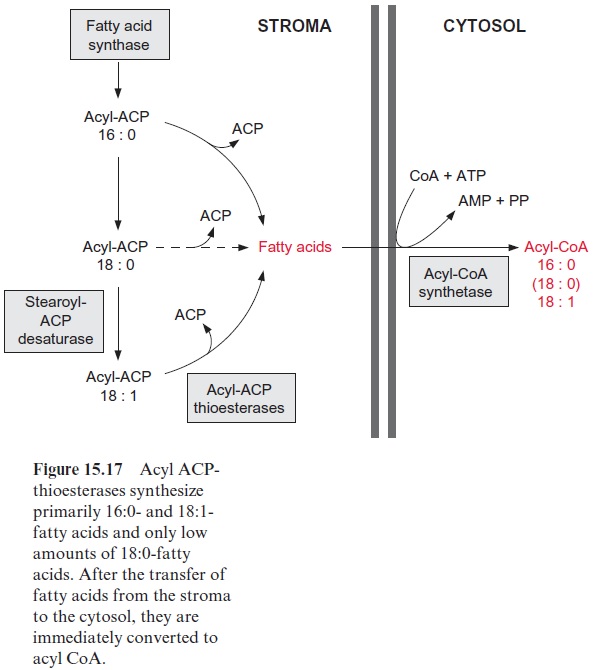
Acyl ACP synthesized as a product of fatty acid synthesis in the plastids serves two purposes
Acyl ACP produced in the plastids has two important functions:
1. It acts as an acyl-donor for the synthesis of plastid membrane lipids. The enzymes of glycerolipid synthesis are in part located in both the inner and outer envelope membranes. Therefore the lipid biosynthesis is a division of labor between these two membranes. In the following text no distinction will be made between the lipid biosynthesis of the inner and outer envelope membranes.
2. For biosynthesis outside the plastids, acyl ACP is hydrolyzed by acyl ACP thioesterases to release fatty acids, which then leave the plastids (Fig. 15.17). It is not known whether this export proceeds via non-specific diffusion or by specific transport. These free fatty acids are imme-diately captured outside the outer envelope membrane by conversion to acyl CoA, a reaction catalyzed by an acyl CoA synthetase with consump-tion of ATP. Since the thioesterases in the plastids hydrolyze primarily 16:0- and 18:1-acyl ACP, and to a small extent 18:0-acyl ACP, the plas-tids mainly provide CoA esters with the acyl residues of 18:1 and 16:0 (also a low amount of 18:0) for lipid metabolism outside the plastids.
Related Topics