Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : The Operating Room Environment
The Operating Room Environment: Medical Gas Systems
Medical Gas Systems
The medical gases commonly used in
operating rooms are oxygen, nitrous oxide, air, and nitrogen. Although
technically not a gas, vacuum exhaust for waste anesthetic gas disposal (WAGD
or scavenging) and surgical suction must also be provided and is considered an
integral part of the medical gas system. Patients are endangered if medical gas
systems, par-ticularly oxygen, are misconfigured or malfunction. The main
features of such systems are the sources of the gases and the means of their
delivery to the operating room. The anesthesiologist must under-stand both
these elements to prevent and detect medical gas depletion or supply line
misconnection. Estimates of a particular hospital’s peak demand determine the
type of medical gas supply system required. Design and standards follow
National Fire Protection Association (NFPA) 99 in the United States and HTM
2022 in the United Kingdom.
SOURCES OF MEDICAL GASES
Oxygen
A reliable supply of oxygen is a
critical requirement in any surgical area. Medical grade oxygen (99% or 99.5%
pure) is manufactured by fractional distilla-tion of liquefied air. Oxygen is
stored as a compressed
gas at room temperature or refrigerated
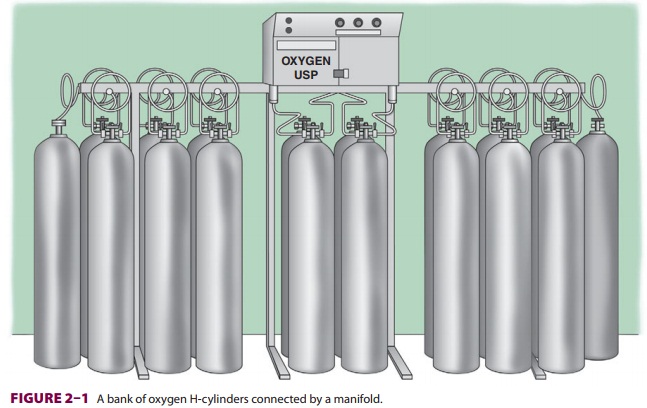
as a liquid. Most small hospitals store oxygen in two separate banks of high-pressure
cylinders (H-cylinders) con-nected by a manifold ( Figure 2–1). Only one bank is
utilized at a time. The number of cylinders in each bank depends on anticipated
daily demand. The manifold contains valves that reduce the cylinder pressure
(approximately 2000 pounds per square inch [psig]) to line pressure (55 ± 5 psig) and auto-matically switch banks when one
group of cylinders is exhausted.
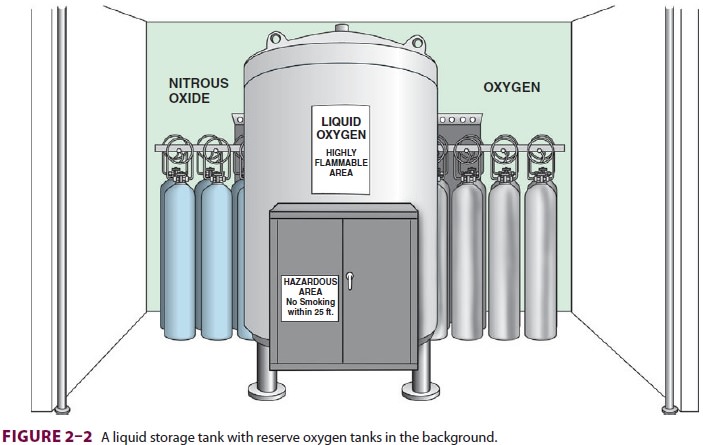
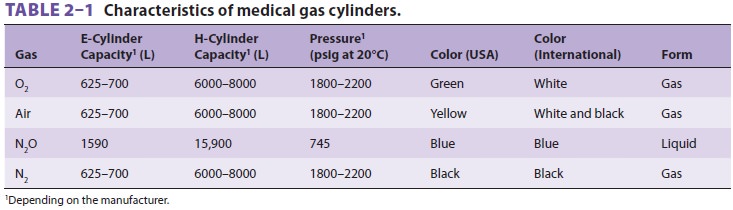
A liquid oxygen storage system (Figure 2–2) is more economical for large hospitals. Liquid oxygen must be stored well below its critical temperature of –119°C because gases can be liquefied by pressure only if stored below their critical temperature. A largehospital may have a smaller liquid oxygen supply or a bank of compressed gas cylinders that can provide one day’s oxygen requirements as a reserve. To guard against a hospital gas-system failure, the anesthesiolo-gist must always have an emergency (E-cylinder) sup-ply of oxygen available during anesthesia.Most anesthesia machines accommodate E-cylinders of oxygen (Table 2–1). As oxygen is expended, the cylinder’s pressure falls in proportionto its content. A pressure of 1000 psig indi-cates an E-cylinder that is approximately halffull and represents 330 L of oxygen at atmospheric pressure and a temperature of 20°C. If the oxygen is exhausted at a rate of 3 L/min, a cylinder that is half full will be empty in 110 min. Oxygen cylinder pressure should be monitored before use and peri-odically during use. Anesthesia machines usually also accommodate E-cylinders for medical air and nitrous oxide, and may accept cylinders of helium. Compressed medical gases utilize a pin index safety system for these cylinders to prevent inadvertent crossover and connections for different gas types. As a safety feature of oxygen E-cylinders, the yoke has integral components made from Wood’s metal. This metallurgic alloy has a low melting point, which allows dissipation of pressure that might otherwise heat the bottle to the point of ballistic explosion. This pressure-relief “valve” is designed to rupture at 3300 psig, well below the pressure E-cylinder walls should be able to with-stand (more than 5000 psig).
Nitrous Oxide
Nitrous oxide is manufactured by heating
ammo-nium nitrate (thermal decomposition). It is almost always stored by
hospitals in large H-cylinders con-nected by a manifold with an automatic
crossover feature. Bulk liquid storage of nitrous oxide is eco-nomical only in
very large institutions.
Because the critical temperature of nitrous oxide (36.5°C) is above room temperature, it can be kept liquefied without an elaborate refrig-eration system. If the liquefied nitrous oxide risesabove its critical temperature, it will revert to its gaseous phase. Because nitrous oxide is not an ideal gas and is easily compressible, this transfor-mation into a gaseous phase is not accompanied by a great rise in tank pressure. Nonetheless, as with oxygen cylinders, all nitrous oxide E-cylinders are equipped with a Wood’s metal yoke to preventvexplosion under conditions of unexpectedly high gas pressure (eg, unintentional overfilling), particu-larly during fires.
Although a disruption in supply is
usually not catastrophic, most anesthesia machines have reserve nitrous oxide
E-cylinders. Because these smaller cyl-inders also contain nitrous oxide in its
liquid state, the volume remaining in a cylinder is not propor-tional to cylinder pressure. By the time the liquid
nitrous oxide is expended and the tank pressure begins to fall, only about 400
L of nitrous oxide remains. If liquid
nitrous oxide is kept at a con-stant temperature (20°C), it will vaporize at
the same rate at which it is consumed and will main-tain a constant pressure
(745 psig) until the liquid is exhausted.
The only reliable way to determine
residual volume of nitrous oxide is to weigh the cylinder. For this reason, the
tare weight (TW), or empty weight, of cylinders containing a liquefied
com-pressed gas (eg, nitrous oxide) is often stamped on the shoulder of the
cylinder. The pressure gauge of a nitrous oxide cylinder should not exceed 745
psig at 20°C. A higher reading implies gauge malfunction, tank overfill (liquid
fill), or a cylinder containing a gas other than nitrous oxide.
Because energy is consumed in the
conversion of a liquid to a gas (the latent heat of vaporization), the liquid
nitrous oxide cools. The drop in tempera-ture results in a lower vapor pressure
and lower cyl-inder pressure. The cooling is so pronounced at high flow rates
that there is often frost on the tank, and pressure regulators may freeze.
Medical Air
The use of air is becoming more frequent
in anesthe-siology as the popularity of nitrous oxide and unnec-essarily high
concentrations of oxygen has declined. Cylinder air is medical grade and is
obtained by blending oxygen and nitrogen. Dehumidified but unsterile air is
provided to the hospital pipeline system by compression pumps. The inlets of
these pumps must be distant from vacuum exhaust vents and machinery to minimize
contamination. Because the critical temperature of air is –140.6°C, it exists
as a gas in cylinders whose pressures fall in proportion to their content.
Nitrogen
Although compressed nitrogen is not
administered to patients, it may be used to drive some operating room
equipment, such as saws, drills, and surgical handpieces. Nitrogen supply
systems either incorpo-rate the use of H-cylinders connected by a manifold or a
wall system supplied by a compressor driven central supply.
Vacuum
A central hospital vacuum system usually
consists of independent suction pumps, each capable of han-dling peak
requirements. Traps at every user location prevent contamination of the system
with foreign matter. The medical-surgical vacuum may be used for waste anesthetic
gas disposal (WAGD) provid-ing it does not affect the performance of the
system. Medical vacuum receptacles are usually black in color with white
lettering. A dedicated WAGD vacuum system is generally required with modern
anesthesia machines. The WAGD outlet may incorporate the use of a suction
regulator with a float indicator. The float should be maintained between the
designated markings. Excess suction may result in inadequate patient
ventilation, and insufficient suction levels may result in the failure to
evaluate WAGD. WAGD receptacles and tubing are usually lavender in color.
Carbon Dioxide
Many surgical procedures are performed
using lapa-roscopic or robotic-assisted techniques requiring insufflation of
body cavities with carbon dioxide, an odorless, colorless, nonflammable and
slightly acidic gas. Large cylinders containing carbon dioxide, such as
M-cylinders or LK-cylinders, are frequently found in the operating room; these
cylinders share a common size orifice and thread with oxygen cylin-ders and can
be inadvertently interchanged.
DELIVERY OF MEDICAL GASES
Medical
gases are delivered from their central sup-ply source to the operating room
through a piping network. Pipes are sized such that the pressure drop across
the whole system never exceeds 5 psig. Gas pipes are usually constructed of
seamless copper tubing using a special welding technique. Internal
contamination
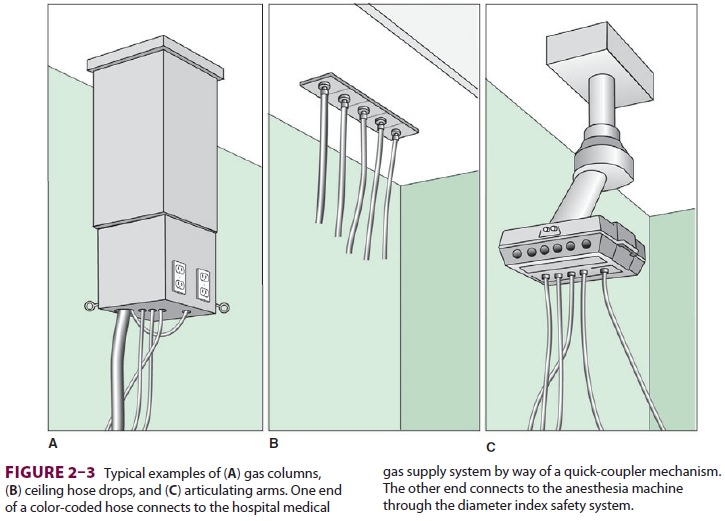
of the pipelines with dust, grease, or water must be avoided. The hospital’s gas
deliv-ery system appears in the operating room as hose drops, gas columns, or
elaborate articulating arms (Figure 2–3). Operating room equipment,
including the anesthesia machine, interfaces with these pipe-line system
outlets by color-coded hoses. Quick-coupler mechanisms, which vary in design
with different manufacturers, connect one end of the hose to the appropriate
gas outlet. The other end connects to the anesthesia machine through a
non-interchangeable diameter index safety system fitting that prevents
incorrect hose attachment.E-cylinders of oxygen, nitrous oxide, and air attach
directly to the anesthesia machine. To discourage incorrect cylinder
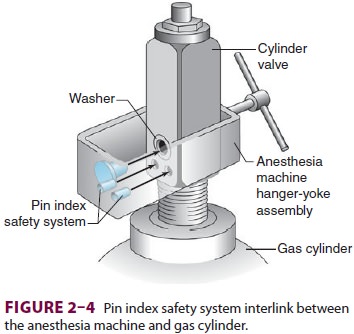
attachments, cylinder manufacturers have adopted a pin index safety system.
Each gas cylinder (sizes A–E) has two holes in its cylinder valve that mate
with correspondingpins in the yoke of the anesthesia machine (Figure 2–4).
The relative positioning of the pins and holes is unique for each gas. Multiple
washers placed between the cylinder and yoke, which prevent proper engagement
of the pins and holes, have unintention-ally defeated this system. The pin
index safety system is also ineffective if yoke pins are damaged or the
cyl-inder is filled with the wrong gas.
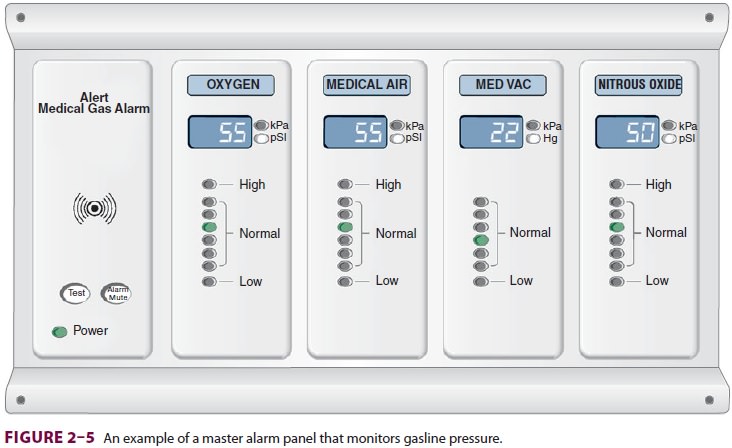
The
functioning of medical gas supply sources and pipeline systems is constantly
monitored by central and area alarm systems. Indicator lights and audible
signals warn of changeover to secondary gas sources and abnormally high (eg,
pressure regulator malfunction) or low (eg, supply depletion) pipeline
pressures (Figure
2–5).
Modern anesthesia machines and anesthetic gas analyzers continuously measure the fraction of inspired oxygen (FiO2). Analyzers have a variable threshold setting for the minimal FiO2 but should be configured to prevent disabling this alarm. The monitoring of FiO2 does not reflect the oxygen con-centration distal to the monitoring port and should not be used to reference the oxygen concentration within devices such as endotracheal tubes or at the distal tip of the tube. Due to gas exchange, flow rates, and shunting a marked difference can exist between the monitored FiO2 and oxygen concentration at the tissue level.
Related Topics