Chapter: Biochemistry: Lipid Metabolism
The Energy Yield from the Oxidationof Fatty Acids
The Energy Yield from the
Oxidationof Fatty Acids
In
carbohydrate metabolism, the energy released by oxidation reactions is used to
drive the production of ATP, with most of the ATP produced in aerobic
processes. In the same aerobic processes—namely, the citric acid cycle and
oxidative phosphorylation—the energy released by the oxidation of acetyl-CoA
formed by β-oxidation of fatty acids can also be used to
produce ATP. There are two sources of ATP to keep in mind when calculating the
overall yield of ATP. The first source is the reoxidation of the NADH and FADH2
produced by the β-oxidation of the fatty acid to acetyl-CoA. The
second source is ATP production from the processing of the acetyl-CoA through
the citric acid cycle and oxidative phosphorylation. We shall use the oxidation
of stearic acid, which contains 18 carbon atoms, as our example.
Eight
cycles of β-oxidation are required to convert one mole of
stearic acid to nine moles of acetyl-CoA; in the process eight moles of FAD are
reduced to FADH2, and eight moles of NAD+ are reduced to
NADH.
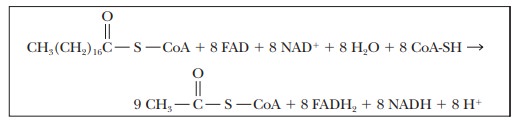
The nine
moles of acetyl-CoA produced from each mole of stearic acid enter the citric
acid cycle. One mole of FADH2 and three moles of NADH are produced
for each mole of acetyl-CoA that enters the citric acid cycle. At the same
time, one mole of GDP is phosphorylated to produce GTP for each turn of the
citric acid cycle.

The FADH2
and NADH produced by β-oxidation and by the citric acid cycle enter
the electron transport chain, and ATP is produced by oxidative phosphorylation.
In our example, there are 17 moles of FADH2 (8 from β-oxidation and 9 from the citric acid cycle); there are also 35
moles of NADH (8 from β-oxidation and 27 from the citric acid cycle).
Recall that 2.5 moles of ATP are produced for each mole of NADH that enters the
electron transport chain, and 1.5 moles of ATP result from each mole of FADH2.
Because 17 - > 1.5 = 25.5 and 35 - > 2.5 = 87.5, we can write the
following equations:

The
overall yield of ATP from the oxidation of stearic acid can be obtained by
adding the equations for β-oxidation, for the citric acid cycle, and for
oxidative phosphorylation. In this calculation, we take GDP as equivalent to
ADP and GTP as equivalent to ATP, which means that the equivalent of nine ATP
must be added to those produced in the reoxidation of FADH2 and
NADH. There are 9 ATP equivalent to the 9 GTP from the citric acid cycle, 25.5
ATP from the reoxidation of FADH2, and 87.5 ATP from the reoxidation
of NADH, for a grand total of 122 ATP.
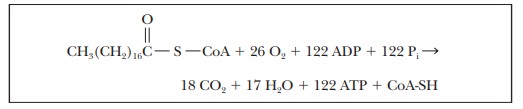
The
activation step in which stearyl-CoA was formed is not included in this
calculation, and we must subtract the ATP that was required for that step. Even
though only one ATP was required, two high-energy phosphate bonds are lost
because of the production of AMP and PPi. The pyrophosphate must be
hydro-lyzed to phosphate (Pi) before it can be recycled in metabolic
intermediates. As a result, we must subtract the equivalent of two ATP for the
activation step. The net yield of ATP becomes 120 moles of ATP for each mole of
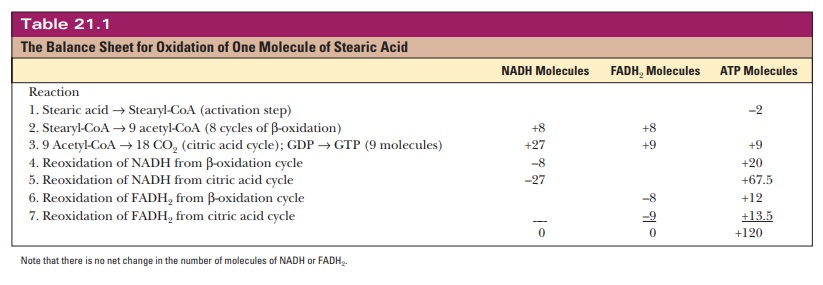
stearic acid that is completely oxidized. See Table 21.1 for a balance sheet.
Keep in mind that these values are theoretical consensus values that not all
cells attain.
As a comparison, note that 32 moles of ATP can be obtained from the com-plete oxidation of one mole of glucose; but glucose contains 6, rather than 18, carbon atoms. Three glucose molecules contain 18 carbon atoms, and a more interesting comparison is the ATP yield from the oxidation of three glucose molecules, which is - > 3 32 = 96 ATP for the same number of carbon atoms. The yield of ATP from the oxidation of the lipid is still higher than that from the carbohydrate, even for the same number of carbon atoms. The reason is that a fatty acid is all hydrocarbon except for the carboxyl group; that is, it exists in a highly reduced state.
A sugar is already partly oxidized because of the presence of
its oxygen-containing groups. Because the oxidation of a fuel leads to the
reduced electron carriers used in the electron transport chain, a more reduced
fuel, such as a fatty acid, can be oxidized further than a partially oxidized
fuel, such as a carbohydrate.

Another
point of interest is that water is produced in the oxidation of fatty acids. We
have already seen that water is also produced in the complete oxida-tion of
carbohydrates. The production of metabolic
water is a common feature of aerobic metabolism. This process can be a
source of water for organisms that live in desert environments. Camels are a
well-known example; the stored lipids in their humps are a source of both
energy and water during long trips through the desert. Kangaroo rats provide an
even more striking example of adaptation to an arid environment. These animals
have been observed to live indefinitely without having to drink water. They
live on a diet of seeds, which are rich in lipids but contain
little water. The metabolic water that kangaroo rats produce is adequate for
all their water needs. This metabolic response to arid conditions is usually
accompanied by a reduced output of urine.
Summary
The complete oxidation of fatty acids by the citric acid cycle and
the elec-tron transport chain releases large amounts of energy.
When we include the reoxidation of NADH and FADH2 from β-oxidation and the citric
acid cycle, we obtain a net yield of 120 ATP for a single molecule of stearic
acid.
Related Topics