Chapter: Biochemistry: Electron Transport and Oxidative Phosphorylation
The Connection between Electron Transportand Phosphorylation
The Connection between Electron
Transportand Phosphorylation
Some of the energy released by the oxidation reactions in the
electron transport chain is used to drive the phosphorylation of ADP. The
phosphorylation of each mole of ADP requires 30.5 kJ = 7.3 kcal, and we have
seen how each reaction catalyzed by three of the four respiratory complexes
provides more than enough energy to drive this reaction, although it is by no
means a direct usage of this energy. A common theme in metabolism is that
energy to be used by cells is converted to the chemical energy of ATP as
needed. The energy-releasing oxidation reactions give rise to proton pumping
and thus to the pH gradient across the inner mitochondrial membrane. In
addition to the pH gradient, a voltage difference across the membrane is
generated by the concentration differences of ions inside and out. The energy
of the electrochemical potential (voltage drop) across the membrane is
converted to the chemical energy of ATP by the coupling process.
What is the coupling factor in oxidative phosphorylation?
A coupling factor is needed to link oxidation and phosphorylation.
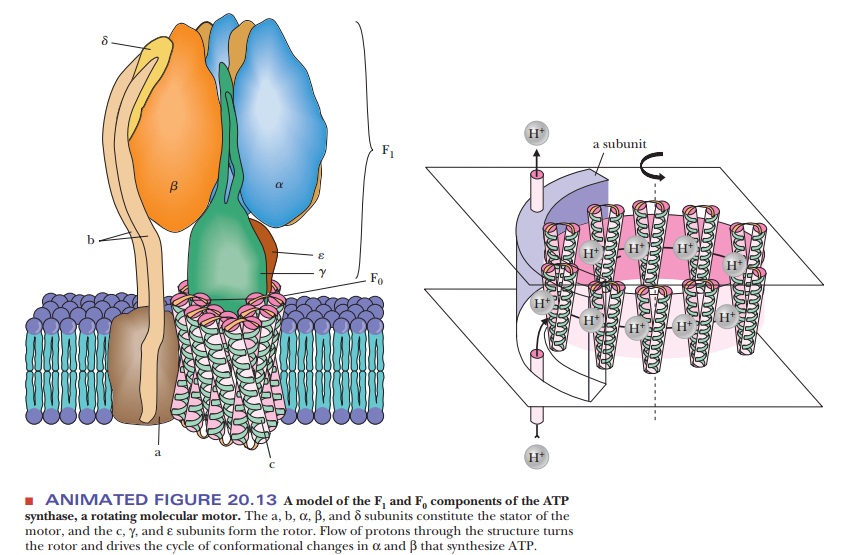
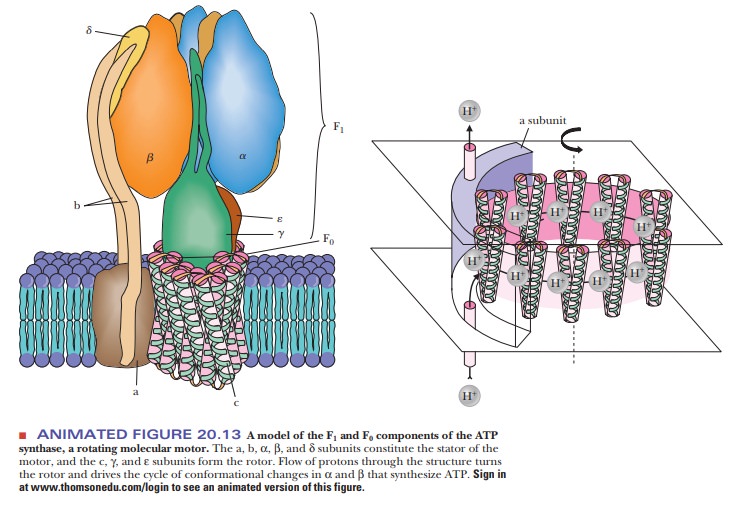
A complex protein oligomer, separate from the electron transport complexes,
serves this function; the complete protein spans the inner mitochondrial
membrane and projects into the matrix as well. The portion of the protein that
spans the membrane is called F0. It consists of three
different kinds of polypeptide chains (a, b, and c), and research is in
progress to characterize it further. The portion that projects into the matrix
is called F1; it consists of five different kinds of polypeptide chains in the
ratio α3β3γδε. Electron micrographs of
mitochondria show the projections into the matrix from the inner mitochondrial
membrane (Figure 20.12). The schematic organization of the protein can be seen
in Figure 20.13. The F1 sphere is the site of ATP
synthesis. The whole protein complex is called ATP synthase. It is also known as mitochondrial ATPase because the
reverse reaction of ATP hydrolysis, as well as phosphorylation, can be
catalyzed by the enzyme. The hydrolytic reaction was discovered before the
reaction of the synthesis of ATP, hence the name. The 1997 Nobel Prize in
chemistry was shared by an American scientist, Paul Boyer of UCLA, and a
British scientist, John Walker of the Medical Research Council in Cambridge,
England, for their elucidation of the structure and mechanism of this enzyme.
(The other half of this prize went to a Danish scientist, Jens Skou, for his
work on the sodium– potassium pump, which also functions as an ATPase.)
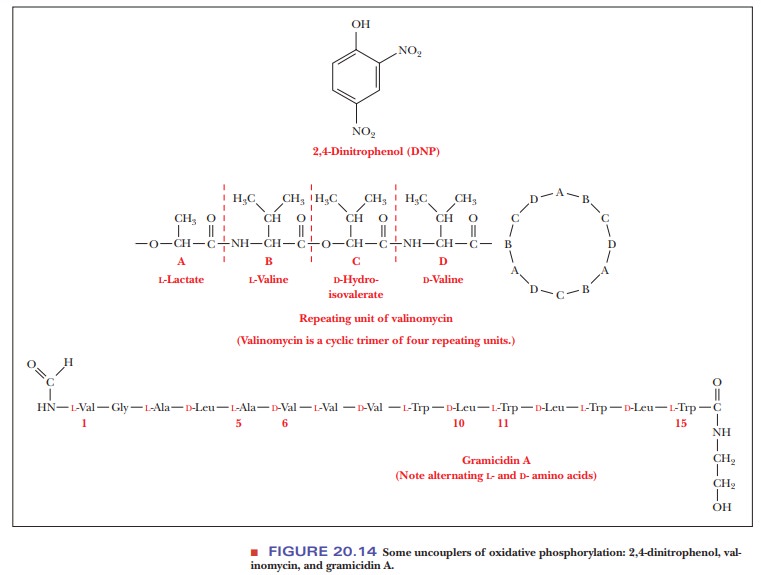
Compounds known as uncouplers inhibit the phosphorylation of ADP without affecting electron transport. A well-known example of an uncoupler is 2,4-dinitrophenol. Various antibiotics, such as valinomycin and gramicidin A, are also uncouplers (Figure 20.14).
When mitochondrial oxidation
processes are operating normally, electron transport from NADH or FADH2 to
oxygen results in the production of ATP. When an uncoupler is present, oxygen
is still reduced to H2O, but ATP is not produced.
If the uncoupler is removed, ATP synthesis linked to electron transport
resumes.
A term called the P/O ratio
is used to indicate the coupling of ATP pro-duction to electron transport. The
P/O ratio gives the number of moles of Pi
consumed in the reaction ADP + Pi - > ATP for each mole of
oxygen atomsconsumed in the reaction 1/2 O2 + 2H +
2e H2O. As we
have already men-tioned, 2.5 moles of ATP are produced when 1 mole of NADH is
oxidized to NAD+. Recall that oxygen is the ultimate acceptor of the electrons from
NADH and that 1/2 mole of O2 molecules (one mole of
oxygen atoms) is reduced for each mole of NADH oxidized. The experimentally
determined P/O ratio is 2.5 when NADH is the substrate oxidized. The P/O ratio
is 1.5 when FADH2 is the substrate oxidized
(also an experimentally determined value). Until recently, biochemists had used
integral values of - > and 2 for the P/O ratios for reoxi-dation of NADH and
FADH2, respectively. The nonintegral consensus values given here clearly
underscore the complexity of electron transport, oxidative phosphorylation, and
the manner in which they are coupled.
Summary
The coupling of electron transport to oxidative phosphorylation
requires a multisubunit membrane-bound enzyme, ATP synthase. This enzyme has a
channel for protons to flow from the intermembrane space into the mitochondrial
matrix.
The proton flow is coupled to ATP production in a process that
appears to involve a conformational change of the enzyme.
Related Topics