Chapter: Introduction to Human Nutrition: Nutrition and Metabolism of Lipids
Terminology of dietary fats
Terminology of dietary fats
Lipids
Like other organic compounds, all lipids are com-posed of a carbon skeleton with hydrogen and oxygen substitutions. Nitrogen, sulfur, and phosphorus are also present in some lipids. Water insolubility is a key but not absolute characteristic distinguishing most lipids from proteins and carbohydrates. There are some exceptions to this general rule, since short- to medium-chain fatty acids, soaps, and some complexlipids are soluble in water. Hence, solubility in a “lipid solvent” such as ether, chloroform, benzene, or acetone is a common but circular definition of lipids.

There are four categories of lipids, as classified by Bloor: simple, compound (complex), derived, and miscellaneous (Table 6.1). Simple lipids are esters of fatty acids with various alcohols such as glycerol or cholesterol. They include triacylglycerols (TAG = neutral fats and oils), waxes, cholesteryl esters, and vitamin A and D esters. Compound lipids are esters of fatty acids in combination with both alcohols and other groups. They include phospholipids, glycolip-ids, cerebrosides, sulfolipids, lipoproteins, and lipo-polysaccharides. Derived lipids are hydrolysis products of simple or compound lipids, including fatty acids, monoacylglycerols and diacylglycerols, straight-chain and ring-containing alcohols, sterols, and steroids. Miscellaneous lipids include some wax lipids, carot-enoids, squalene, and vitamins E and K.
Saturated and unsaturated fatty acids
The main components of dietary fat or lipids are fatty acids varying in length from one to more than 30 carbons. They are carboxylic acids with the structure RCOOH, where R is hydrogen in formic acid, CH3 in acetic acid, or else a chain of one to over 30 CH2 groups terminated by a CH3 group. The various names for individual fatty acids (common, official) and their abbreviations are complicated, and the use of one or other form is somewhat arbitrary. The basic rule for the abbreviations is that there are three parts: number of carbons, number of double bonds, and position of the first double bond. Thus, the common dietary saturated fatty acid palmitate is 16:0 because it has 16 carbons and no double bonds. The common dietary polyunsaturated fatty acid linoleate is 18:2n-6 because it has 18 carbons, two double bonds, and the first double bond is at the sixth carbon from the methyl-terminal (n-6). Beyond six carbons in length, most fatty acids have an even number of carbons (Table 6.2). Older fatty acid ter-minology referring to saturated or unsaturated carbons in lipids that still occasionally appears includes: aliphatic (a saturated carbon), olefinic (an unsaturated carbon), allylic (a saturated carbon adja-cent to an unsaturated carbon), and doubly allylic carbon (a saturated carbon situated between two unsaturated carbons).

Lengthening of the chain an the introduction of additional double bonds beyond the first one occur from the carboxyl-terminal. The presence of one or more double bonds in a fatty acid defines it as “unsat-urated,” compared with a saturated fatty acid which contains no double bonds. A saturated fatty acid gen-erally occupies less space than an equivalent chain length unsaturated fatty acid (Figure 6.1). Double bonds allow for isomerization or different orientation (cis or trans) of the adjoining carbons across the double bond (Figure 6.2). In longer chain fatty acids, double bonds can also be at different positions in the molecule. Hence, unsaturation introduces a large amount of structural variety in fatty acids and the resulting lipids.
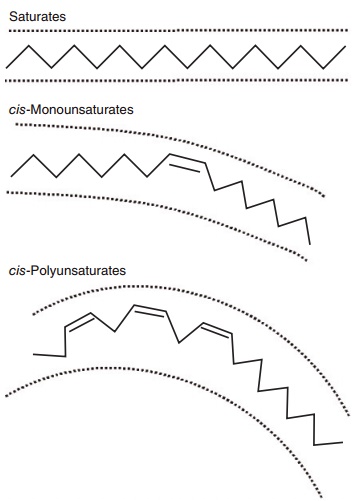
Figure 6.1 Stick models illustrating the basic structural differences between saturated, cis-monounsaturated, and cis-polyunsaturated fatty acids. As shown in two dimensions, the increasing curvature caused by inserting one or more double bonds increases the area occupied by the fatty acid. The physical area occupied by unsaturated fatty acids is further accentuated in three dimensions because esteri-fied fatty acids rotate around the anchored terminal.
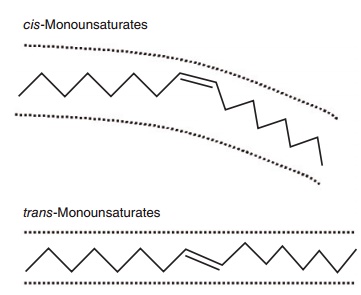
Figure 6.2 Stick models comparing a cis- with a trans-unsaturated fatty acid. A cis-unsaturated double bond creates a U-shaped space and confers curvature to the molecule because, relative to the longi-tudinal axis of the fatty acid, the two hydrogens at the double bond are on the same side of the molecule. A trans-unsaturated double bond does not confer curvature to the molecule because the hydrogens are on opposite sides of the double bond. A trans-double bond therefore tends to give the fatty acid physicochemical properties more like that of a saturated fatty acid.
Short- and medium-chain fatty acids
Short-chain fatty acids (less than eight carbons) are water soluble. Except in milk lipids, they are not commonly esterified into body lipids. Short-chain fatty acids are found primarily in dietary prod ucts containing ruminant milk fat.
Hence, although they are produced in relatively large quantities from the fermentation of undigested carbohydrate in the colon, as such, they do not become part of the body lipid pools. Medium-chain fatty acids (8–14 carbons) arise as intermediates in the synthesis of long-chain fatty acids or by the consumption of coconut oil or medium-chain TAG derived from it. Like short-chain fatty acids, medium-chain fatty acids are present in milk but they are also rarely esterified into body lipids, except when consumed in large amounts in clinical situations requiring alternative energy sources. Medium-chain fatty acids (8–14 carbons) are rare in the diet except for coconut and milk fat.
Long-chain saturated and monounsaturated fatty acids
Long-chain fatty acids (>14 carbons) are the main constituents of dietary fat. The most common satu-rated fatty acids in the body are palmitate and stea-rate. They originate from three sources: directly from the diet, by complete synthesis from acetyl-coenzyme
Palmitate and stearate are important membrane constituents, being found in most tissue phospholip-ids at 20–40% of the total fatty acid profile. Brain membranes contain 20- to 24-carbon saturates that, like palmitate and stearate, are synthesized within the brain and have little or no access to the brain from the circulation. The normal membrane content of long-chain saturates can probably be sustained without a dietary source of these fatty acids. Com-pared with all other classes of dietary fatty acid, espe-cially monounsaturated or polyunsaturated fatty acids, excess intake or synthesis of long-chain satu-rates is associated with an increased risk of cardiovas-cular disease.
The most common long-chain cis-monounsatu-rated fatty acids in diet and in the body are oleate (18:1n-9) and palmitoleate (16:1n-7), with the former predominating by far in both the body’s storage and membrane lipids. As with stearate, most oleate in the human body appears to be of dietary origin. Hence, although humans have the capacity to desaturate stearate to oleate, dietary oleate is probably the domi-nant source of oleate in the body. Only plants can further desaturate oleate to linoleate and again to α-linolenate. As with saturates of >18 carbons in length, 20-, 22-, and 24-carbon monounsaturates derived from oleate are present in specialized membranes such as myelin.
Polyunsaturated fatty acids (PUFAs)
Linoleate and α-linolenate are the primary dietary cis-polyunsaturated fatty acids in most diets. Neither can be synthesized de novo (from acetate) in animals so are ‘essential’ fatty acids. They can be made by chain elongation from the two respective 16-carbon precursors, hexadecadienoate (16:2n-6) and hexadecatrienoate (16:3n-3), which are found in common edible green plants at up to 13% of total fatty acids. Hence, significant consumption of green vegetables will provide 16-carbon polyunsaturates that contribute to the total available linoleate and α-linolenate.
Linoleate is the predominant polyunsaturated fatty acid in the body, commonly accounting for 12–15% of adipose tissue fatty acids. In the body’s lean tissues there are at least three polyunsaturates present in amounts >5% of the fatty acid profile (linoleate, ara-chidonate, docosahexaenoate). In addition, at least two other biologically active polyunsaturates are present in body lipids [dihomo-γ-linolenate (20:3n-6) and eicosapentaenoate (20:5n-3)], although usually in amounts between 1% and 3% of total fatty acids. Marine fish are the richest source of 20- to 22-carbon polyunsaturates. α-Linolenate and its precursor, hexadecatrienoate (16:3n-3), are the only n-3 polyun-saturates in common terrestrial plants.
Hydrogenated and conjugated fatty acid isomers
The introduction of unsaturation with one double bond creates the possibility of both positional and geometric isomers in fatty acids. Among long-chain unsaturated fatty acids, positional isomers exist because the double bond can be introduced into several different locations, i.e., 18:1n-7, 18:1n-9, 18:1n-11, etc. Geometric isomers exist because the two remaining hydrogens at each double bond can be opposite each other (trans) or on the same side of the molecule (cis; Figure 6.2). Thus, there is cis-18:1n-9 (oleate) and trans-18:1n-9 (elaidate), and so on for all unsaturated fatty acids, with the combinations mounting exponentially as the number of double bonds increases.
Trans isomers of monounsaturated or polyunsatu-rated fatty acids arise primarily from partial hydroge-nation during food processing of oils, but some also occur naturally in ruminants. The number of trans isomers increases with the number of double bonds, so there is only one trans isomer of oleate but there are three trans isomers of linoleate and seven of α-linolenate. Virtually all naturally occurring polyun-saturated fatty acids have double bonds that are methylene interrupted, i.e., have a CH2 group between the two double bonds. However, methylene interrup-tion between double bonds can be lost, again, through food processing, and the bonds moved one carbon closer together, becoming conjugated. Thus, the double bonds in linoleate are at the 9–10 and 11–12 carbons, but in conjugated linoleate they are at the 9–10 carbons and the 11–12 carbons. Some degree of further desaturation and chain elongation can occur in conjugated fatty acids, but much less than with methylene-interrupted polyunsaturates. Thus, conju-gated linoleate is the main conjugated fatty acid that has attracted considerable attention with respect to its potential role in nutritional health.
Fats and oils
Fats are esters of fatty acids with glycerol (Table 6.1). They usually occur as triesters or triacylglycerols (TAGs), although monoacylglycerols and diacylglyc-erols occur during fat digestion and are used in food processing. Most common dietary fats contain a mixture of 16- to 18-carbon saturated and unsatu-rated fatty acids. By convention, fats that are liquid at room temperature are called oils, a feature arising from their lower proportion of saturated (straight-chain) and higher proportion of unsaturated (bent-chain) fatty acids. Unsaturated fatty acids usually have a lower melting point; this facilitates liquefaction of the fats of which they are a component. TAGs of animal origin are commonly fats, whereas those of fish or plant origin are usually oils. Animal fats and fish oils frequently contain cholesterol, whereas plant oils do not contain cholesterol but usually contain other “phyto” sterols.
TAGs are primarily used as fuels, so dietary fats (mostly TAGs) are commonly associated with energy metabolism rather than with structural lipids found in membranes. However, membrane lipids as well as TAGs are extracted with lipid solvents used to determine the fat content of foods, tissues, or plant material. Hence, because organs such as brain are rich in membrane phospholipids, when the total lipids are extracted to determine the organ’s chemical composition, these organs are said to have a certain fat content. On a chemical basis this is true, but this description often misconstrues the nature of the lipid because the brain in particular contains virtually no TAG.
Phospholipids
Phospholipids contain two nonpolar, hydrophobic acyl tail groups and a single functional head group that is polar and hydrophilic. Hence, they are rela-tively balanced amphiphilic lipids and, in this capac-ity, are crucial components of biological membranes.
The head groups contain phosphorus and amino acids (choline, serine, ethanolamine), sugars (inosi-tol), or an alcohol (glycerol). Phosphatidylcholine (lecithin) is the most abundant phospholipid in animal tissues but phosphatidylglycerols (glycosides) predominate in plant lipids. Phospholipids contain-ing a fatty acid amide are sphingolipids. Various phos-pholipases can hydrolyze the acyl groups or head group during digestion or metabolism.
One of the outstanding characteristics that make phospholipids suitable as major constituents of bio-logical membranes is that, in water, they naturally aggregate into spherical or rod-like liposomes or ves-icles, with the hydrophilic portion facing outwards and the hydrophobic portion facing inwards (Figure 6.3). Changing the constituent acyl groups from satu-rated to polyunsaturated changes the fluidity of these aggregates because of the greater amount of space
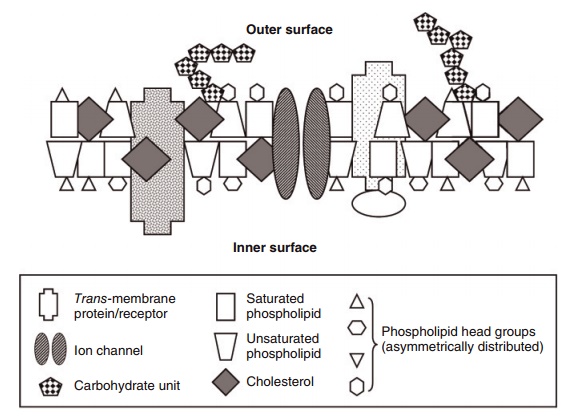
Figure 6.3 Simplified schematic view of a membrane bilayer. The main components are proteins, free cholesterol, phospholipids, and carbohy-drates. There are many different proteins with a myriad of shapes, membrane distribution, and functions, of which three are illustrated. Membrane phospholipids principally help to create the bilayer. They have four types of “head groups” (choline, ethanolamine, serine, and inositol) that are located at or near the membrane’s two surfaces. The two fatty acids in phospholipids are mixtures of 16- to 22-carbon saturates, monounsaturates, and polyunsaturates in all combinations, with those rich in unsaturated fatty acids occupying more space; hence, their trapezoid shape compared with the narrower, rectangular shape of the more saturated phospholipids. Free cholesterol represents 30–40% of the lipid in most membranes. The many different carbohydrates are on the membrane’s surfaces and are bound to lipids and/or proteins in the membrane.
occupied by more unsaturated fatty acids. At inter-faces between non-miscible polar and non-polar sol-vents, phospholipids also form a film or monolayer.
Sterols
The main sterol of importance in human nutrition is cholesterol. It has multiple roles including being:
● a vital component of biological membranes
● a precursor to bile salts used in fat digestion
● a precursor to steroid hormones.
Sterols are secondary alcohols belonging to the poly-isoprenoids or terpinoids (terpenes), which have a common precursor, isopentenyl diphosphate. Other members of the terpinoids include squalene, carot-enoids, and dolichols. Bacteria appear to be the only life forms not containing cholesterol. Sterols have a common cyclopentano(a)perhydrophenanthrene skeleton with different substitutions giving rise to the multiple sterols and steroids.
Related Topics