Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Individuals, populations, and assemblages
Synthesis: what determines assemblage structure among coral reef fishes?
Synthesis: what determines assemblage structure among coral reef fishes?
Coral reefs contain more species of fishes than any other habitat. Almost 700 shallow water species occur in the Caribbean, and Indo-Pacific reefs are home to more than 3000 species (see Marine zoogeographic regions). This incredible diversity (plus colorful fishes and clear, warm water) has understandably drawn the attention of fish ecologists and led to some surprisingly emotional controversies. Probably the most divisive debate concerns the maintenance of this high diversity. How does a coral reef support so many different fishes? What determines the number of species and individuals that will occur on a reef? Can one predict what kinds of fishes (both taxonomically and ecologically) will occur on different reefs or on the same reef at different times? Do the processes that determine species abundance and diversity in one tropical ocean apply to reefs in another ocean? These questions have more than theoretical value. Understanding spatial and temporal patterns of recruitment and the factors that determine the success of recruits can influence fisheries management and conservation practices such as seasonal closures, protected area and species protection, and artifi cial reef design and placement (Beets 1989; Bohnsack et al. 1994; NRC 2001).
These and related topics have nurtured what has become known as the “stochastic–deterministic debate”. The terms refer to the two general processes affecting the maintenance of diversity. Stochastic processes are largely random in operation. An extreme adherent of the stochastic school would argue that chance events affecting planktonic larvae and newly recruited juveniles play too large a role for us to be able to predict species composition. The ocean is a huge place with very little shallow water habitat. A larval fish that was not eaten or that did not starve to death must also be lucky enough to encounter a reef during the brief period when it is competent to settle. The larva is also likely to get eaten by zooplanktivorous predators that abound on reefs, and it must finally find a suitable, unoccupied site in which to settle. These chance events, which are further influenced by unpredictable storms (see Extreme weather), reduce the accuracy with which we can predict the actual species and abundances of fishes that will occur on a specific reef, beyond knowing what occurs in a general geographic region.
An extreme determinist, in contrast, would argue that biological interactions such as competition, predation, and symbiosis have led to an evolutionarily fine-tuned assemblage of species. Each species has a well-defined ecological niche that includes competitive, cooperative, and predator– prey interactions with other species. Hence new recruits will become established depending on which residents already occur at a site and which niches are unfilled at a given time (a similar debate has developed around the question of assemblage stability in temperate stream fishes; see for example Grossman et al. (1982, 1985) “versus” Herbold (1984), Rahel et al. (1984), and Yant et al. (1984); reviewed by Strange et al. (1993)).
Few fish ecologists would adhere to either extreme view. The debate, after accounting for differences in methodology and study site, really boils down to an argument as to which phenomenon – chance events or biological interactions – plays the larger role in determining species composition at different locales. Observational and experimental studies in the Caribbean and Pacific, beginning largely with the work of Sale (1978) and Smith (1978), have led to different conclusions. Observational studies emphasize comparisons of underwater counts of individuals at several sites in one reef area, or at the same locale at several different times or after a hurricane strikes an area (in the latter situation, one must be “fortunate” enough to have been conducting work at the site before the hurricane struck). Experimental studies usually involve removal of individuals from small coral heads or patch reefs, or the addition of small patches of reef habitat followed by monitoring of recruitment and recolonization.
Reef fish assemblages are usually defined in terms of the adults present, but replacement of adults is seldom by other adults. When an adult is removed, either experimentally by a researcher or naturally by a predator, it is usually replaced by a newly settled (recruited) juvenile or a slightly older colonist. The question of interest then becomes one of whether adult population dynamics are determined by events before settlement (i.e., during the planktonic phase), during recruitment (i.e., by settling larvae), or after recruitment (i.e., due to interactions among juveniles, adults, and their competitors and predators) (Fig. 24.9).
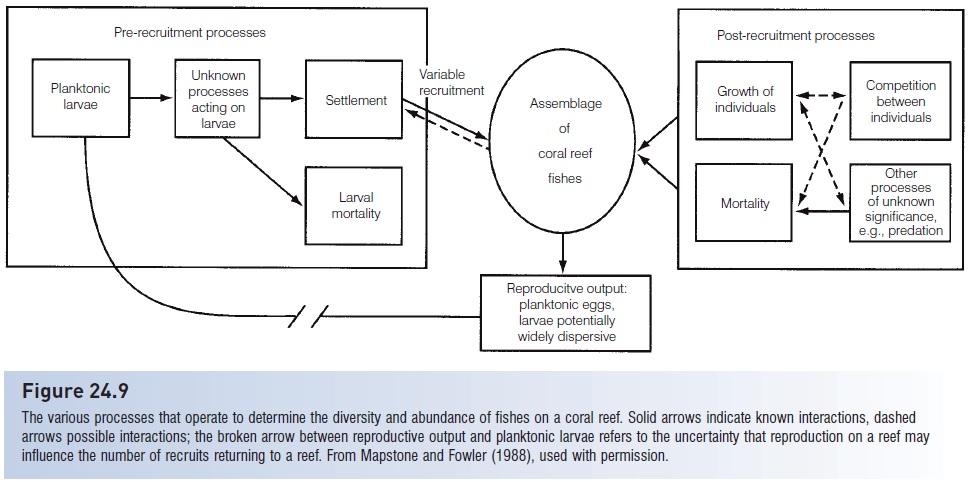
Figure 24.9
The various processes that operate to determine the diversity and abundance of fishes on a coral reef. Solid arrows indicate known interactions, dashed arrows possible interactions; the broken arrow between reproductive output and planktonic larvae refers to the uncertainty that reproduction on a reef may influence the number of recruits returning to a reef. From Mapstone and Fowler (1988), used with permission.
Some workers emphasize the importance of events and interactions in the plankton in determining which species populate a reef (Doherty & Williams 1988; Wellington & Victor 1988). This view developed from observations of similar, unexploited reefs in close proximity to one another that contained dissimilar species assemblages. Also, experimentally increased food and refuge availability on a reef does not necessarily lead to an increase in fish numbers at a site. Hence reefs may contain fewer individuals than they can theoretically support, i.e., they often exist below their carrying capacity. These findings suggest that adult populations may be limited by the number of larvae available to settle in the area, the so-called recruitment (or settlement) limitation hypothesis. Additional evidence of recruitment limitation includes differences in year class strength on a reef and the rarity of larvae of some species.
The alternative view – the habitat limitation or interactive hypothesis – proposes that appropriate habitat is limiting or that post settlement biological interactions (predation, competition) determine the kinds and abundances of fishes on a reef, regardless of larval abundance. The habitatlimited or interactive scenario depicts a reef at carrying capacity, one that is less able to replace fish removed through fishing. Evidence includes superabundant larvae around reefs, reefs packed with recruits, and rates of predation that exceed 99% during the first year postsettlement (e.g., Shulman & Ogden 1987; reviewed in Roberts 1996; Hixon 1998; Hobson et al. 2001; Levin & Grimes 2002).
Many factors can affect larval abundance, regardless of numbers or habitat availability. These include predation on larvae by vertebrate and invertebrate planktivores, food availability for larvae, and dispersal away from appropriate settling areas. It is well known that mortality is the most likely fate awaiting a planktonic larva; most studies estimate that more than 99% of larvae die or are eaten before they settle (see above, Life histories and reproductive ecology). Thus antipredator, food-getting, and active dispersal adaptations of larvae themselves may be critical. Adults can also improve their offspring’s chances of making it through the planktonic filter by spawning at times and places that minimize dispersal away from home reefs, which reduces the area over which larvae must search for an appropriate settling habitat. Carefully chosen spawning locales can also place larvae where planktonic food tends to be concentrated (but also where predators on planktonic fish larvae also abound) (Johannes 1978).
Periodically, vast numbers of larvae that are ready to settle can be found around reefs, indicating that habitat limitation can in fact be important at times (Victor 1991; Kaufman et al. 1992). Under such circumstances, conditions that prevail in the settlement area may determine the success of recruits and the ultimate species composition in an area. Several studies have shown that small artifi cial reefs placed in shallow water attract large numbers of recently settled larvae to areas that were previously devoid of larvae, suggesting that appropriate, unoccupied habitat can limit recruitment. But regardless of habitat availability, previous occupation of the habitat, known as priority effects, may be crucial in determining whether larvae settle successfully. If the first occupants of a coral patch are herbivores or small planktivores, a variety of larvae will follow and take up residence. However, if the first settlers are predators such as moray eels, squirrelfishes, grunts, snappers, or groupers, later recruitment will be greatly reduced as these small predators eat incoming fish larvae (Beets 1997; Tupper & Juanes 1999) (Fig. 24.10). Which larvae settle first is governed largely by chance because of the unpredictable nature of planktonic existence. Once larvae settle, their impact on later settlers is fairly predictable and depends
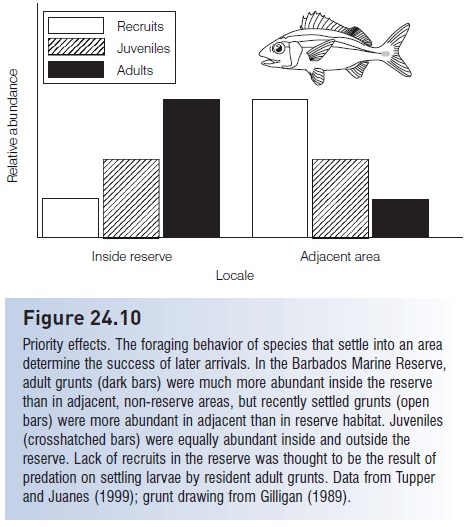
Figure 24.10
Priority effects. The foraging behavior of species that settle into an area determine the success of later arrivals. In the Barbados Marine Reserve, adult grunts (dark bars) were much more abundant inside the reserve than in adjacent, non-reserve areas, but recently settled grunts (open bars) were more abundant in adjacent than in reserve habitat. Juveniles (crosshatched bars) were equally abundant inside and outside the reserve. Lack of recruits in the reserve was thought to be the result of predation on settling larvae by resident adult grunts. Data from Tupper and Juanes (1999); grunt drawing from Gilligan (1989).
A growing body of knowledge has changed our perception of larval life and behavior. The classical view was of passive larvae carried by ocean currents, settling when they reached some critical stage of competency. If a larva happened to be over appropriate habitat at that stage, its chances were good. If it was somewhere less favorable, such as over great ocean depths, then it was game over. We now know that larvae are much more active than this in their settling activities (see Getting from here to there: larval transport mechanisms). Larvae are attracted to reef areas by both sounds and smells emitted by reefs, and move actively toward appropriate stimuli (Atema et al. 2002; Kingsford et al. 2002; Tolimieri et al. 2004; Gerlachet al. 2007). Once over a reef, larvae (or more accurately transitional juveniles) show strong habitat preferences that differ among species; some larvae will settle and then ascend back into the water column if conditions are inappropriate. Larval settlement is therefore not a parachute drop but more of a bungee jump (Kaufman et al. 1992; Lecchini 2005). Maintenance of high diversity on a reef demands protection of not just adult habitats but also of settlement habitats, which are often different from and far removed from adult habitats.
A larva that settles successfully onto a patch of reef and transforms into a juvenile is by no means guaranteed a long and productive life. Biological interactions involving predation, competition, and cooperation, and their interactions, can have a strong impact on individual success. Mortality rates remain high once larvae have settled; 25% of recruits may die in the first 5 days after settlement, but this rate falls to <10% after 6 days and continues to decrease thereafter (Doherty & Sale 1986). Although the rate may slow, the numbers killed remains high, varying between 65% and 99.9% during the first year after settlement (Sweatman 1984; Shulman & Ogden 1987). Mortality may also be density dependent, increasing as population size increases, as happens when large numbers of juvenile humbug damselfishes, Dascyllus spp., actively compete for preferred nighttime resting locales among branching corals. Less aggressive individuals are displaced to riskier locations,
where they are subject to predation by crepuscular/ nocturnal predators such as squirrelfishes. Again, diversity maintenance on a reef requires protection of both daytime feeding and nighttime resting habitats, which again may differ (Holbrook & Schmitt 2002; see also Hixon & Carr 1997).
Larvae may settle more successfully in isolated habitats away from major concentrations of larger fishes and then move later to the more extensive reef habitats. This may be one reason that back-reef areas, mangroves, and seagrass beds are often the preferred habitat for the juveniles of many “reef ” species (Manson et al. 2005; Adams et al.2006; Pollux et al. 2007). The move to the reef itself is also full of perils, as predators tend to patrol the edges of reefs and catch prey moving across refugeless zones (Shulman 1985a, 1985b). For species that have symbiotic relationships with invertebrates, such as anemonefishes, carapid pearlfishes, cardinalfishes, and many gobies, successful location of an unoccupied, species-specific host is probably a good guarantor of survival, but such hosts may be in limited supply. An added complication is the cannibalism that occurs if the species-specific host is already occupied by an adult of the larva’s species (e.g., Tyler et al. 1992; Mutualism and commensalism).
One possibly infl uential difference between major oceans is the size of the eggs produced by residents (Thresher 1982). On average, egg sizes are smaller in the western Atlantic than the western Pacific. Smaller eggs imply greater fecundity among Atlantic species, which could lead to greater reproductive output in the western Atlantic. Assuming comparable larval mortality rates, more larvae mean more potential competition for space among recruits, tipping the scales in favor of a stronger role for deterministic interactions among Atlantic coral reef fishes than their Pacific relatives.
The bottom line to this discussion is that arguments about the relative importance of stochastic and deterministic factors and their influences on reef fish assemblage structure and dynamics oversimplify the situation. Both types of factor come into play and have different levels of influence at different times and in different places. Random events in the plankton undoubtedly influence which larvae will survive, and whether the larva settles successfully depends on whether space is available for it on the reef. Space becomes available in part as a chance result of predation and storm disturbance. But larvae, juveniles, and adults often have specific habitat preferences, as shown by the fairly distinctive zones that occur on most coral reefs (lagoonal, patch reef, back reef, reef crest, shallow and deep reef front) and the fairly predictable assemblages of species found in each zone (e.g., Nanami et al. 2005; Ashworth et al. 2006). Non-random competitive, predatory, and mutualistic interactions affect the suitability of a site, the survivorship of its inhabitants, and ultimately its availability for new recruits and colonists. Space availability may depend on the guild of a fish that has been removed from the reef. We can predict that certain guilds are likely to be present on a reef, but we cannot predict which members of that guild will be present. The importance then of the different factors is going to vary from time to time and place to place.
Related Topics