Chapter: 11th Physics : UNIT 7 : Properties of Matter
Surface Tension by capillary rise method
Surface
Tension by capillary rise method
The
pressure difference across a curved liquid-air interface is the basic factor
behind the rising up of water in a narrow tube (influence of gravity is
ignored). The capillary rise is more dominant in the case of very fine tubes.
But this phenomenon is the outcome of the force of surface tension. In order to
arrive a relation between the capillary rise (h) and surface tension (T),
consider a capillary tube which is held vertically in a beaker containing
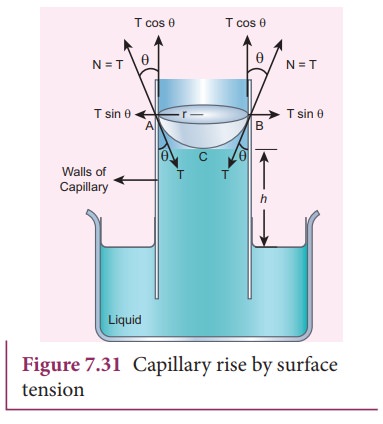
water; the water rises in the capillary tube to a height h due to surface tension (Figure 7.31).
![]()
![]() The surface tension force FT, acts along the tangent at the point of contact downwards
and its reaction force upwards. Surface tension T, is resolved into two components i) Horizontal component TŌĆåsin╬Ė and ii) Vertical
component TŌĆåcos╬Ė acting upwards, all
along the whole circumference of the meniscus.
The surface tension force FT, acts along the tangent at the point of contact downwards
and its reaction force upwards. Surface tension T, is resolved into two components i) Horizontal component TŌĆåsin╬Ė and ii) Vertical
component TŌĆåcos╬Ė acting upwards, all
along the whole circumference of the meniscus.
Total
upward force
=
(TŌĆåcos╬Ė) (2ŽĆr) = 2ŽĆrTŌĆåcos╬Ė
where
╬Ė is the angle of contact, r is the
radius of the tube. Let Žü be the density of water and h be the height to which the liquid rises inside the tube. Then,
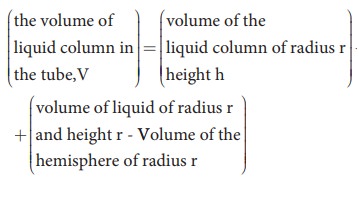
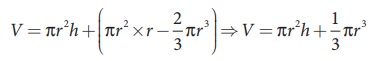
The
upward force supports the weight of the liquid column above the free surface,
therefore,
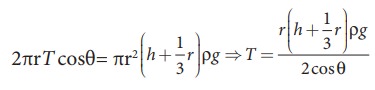
If
the capillary is a very fine tube of radius (i.e., radius is very small) then r/3
can be neglected when it is compared to the height h. Therefore,
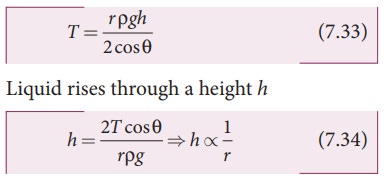
This
implies that the capillary rise (h)
is inversely proportional to the radius (r)
of the tube. i.e, the smaller the radius of the tube greater will be the
capillarity.
EXAMPLE 7.12
Water
rises in a capillary tube to a height of 2.0cm. How much will the water rise
through another capillary tube whose radius is one-third of the first tube?
Solution
From
equation (7.34), we have
h
ŌłØ 1/r ŌćÆhr =constant
Consider
two capillary tubes with radius r1
and r2 which on placing in
a liquid, capillary rises to height h1
and h2, respectively.
Then,
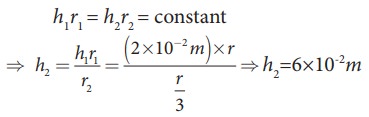
EXAMPLE 7.13
Mercury
has an angle of contact equal to 140┬░ with soda lime glass. A narrow tube of
radius 2ŌĆåmm, made of this glass is
dipped in a trough containing mercury. By what amount does the mercury dip down
in the tube relative to the liquid surface outside?. Surface tension of mercury
T=0.456 NŌĆåm-1; Density of mercury Žü = 13.6 ├Ś 103 kg m-3
Solution
Capillary
descent,
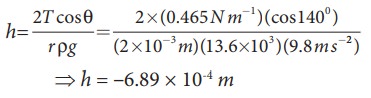
where,
negative sign indicates that there is fall of mercury (mercury is depressed) in
glass tube.
Related Topics