Chapter: 11th Physics : UNIT 7 : Properties of Matter
Surface Tension: Intermolecular forces
SURFACE
TENSION
Intermolecular
forces
Different
liquids do not mix together due to their physical properties such as density,
surface tension force, etc. For example, water and kerosene do not mix
together. Mercury does not wet the glass but water sticks to it. Water rises up
to the leaves through the stem. They are mostly related to the free surfaces of
liquids. Liquids have no definite shape but have a definite volume. Hence they
acquire a free surface when poured into a container. Therefore, the surfaces
have some additional energy, called as surface energy. The phenomenon behind
the above fact is called surface tension. Laplace and Gauss developed the
theory of surface and motion of a liquid under various situations.
The
molecules of a liquid are not rigidly fixed like in a solid. They are free to
move about. The force between the like molecules which holds the liquid
together is called ‘cohesive force’.
When the liquid is in contact with a solid, the molecules of the these solid
and liquid will experience an attractive force which is called ‘adhesive force’.
These
molecular forces are effective only when the distance between the molecules is
very small about 10-9 m (i.e., 10 Ã…). The distance through which the influence
of these molecular forces can be felt in all directions constitute a range and
is called sphere of influence. The
forces outside this range are rather
negligible.
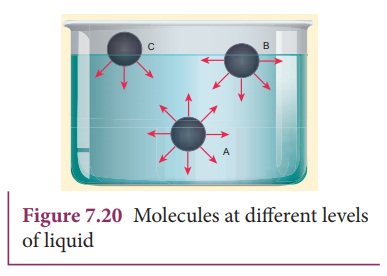
Consider
three different molecules A, B, and C in a given liquid as shown in Figure
7.20. Let a molecule ‘A’ be considered well inside the liquid within the sphere
of influence. Since this molecule interacts with all other molecules in all
directions, the net force experienced by A is zero. Now consider a molecule ‘B’
in which three-fourth lies below the liquid surface and one–fourth on the air.
Since B has more molecules towards its lower side than the upper side, it
experiences a net force in the downward direction. In a similar way, if another
molecule ‘C’ is chosen on the liquid surface (i.e, upper half in air and lower
half in liquid), it experiences a maximum downward force due to the
availability of more number of liquid molecules on the lower part. Hence it is
obvious that all molecules of the liquid that falls within the molecular range
inside the liquid interact with the molecule and hence experience a downward
force.
When
any molecule is brought towards the surface from the interior of the liquid,
work is done against the cohesive force among the molecules of the surface.
This work is stored as potential energy in molecules. So the molecules on the
surface will have greater potential energy than that of molecules in the
interior of the liquid. But for a system to be under stable equilibrium, its
potential energy (or surface energy) must be a minimum. Therefore, in order to
maintain stable equilibrium, a liquid always tends to have a minimum number of
molecules. In other words, the liquid tends to occupy a minimum surface area. This
behaviour of the liquid gives rise to surface tension.
Examples for surface tension.
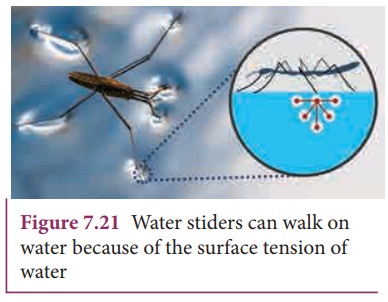
Water
bugs and water striders walk on the surface of water (Figure 7.21). The water
molecules are pulled inwards and the surface of water acts like a springy or
stretched membrane. This balance the weight of water bugs and enables them to
walk on the surface of water. We call this phenomenon as surface tension.
The
hairs of the painting brush cling together when taken out of water. This is
because the water films formed on them tends to contract to a minimum area
(Figure 7.22).

Activity 1:Needle floats on water surface
Take
a greased needle of steel on a piece of blotting paper and place it gently over
the water surface. Blotting paper soaks water and soon sinks down but the
needle keeps floating.
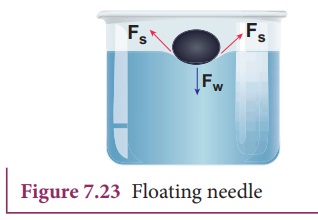
The
floating needle causes a little depression; the forces F, due to the surface
tension of the curved surface are inclined as shown in Figure 7.23. The
vertical components of these two forces support the weight of the needle. Now
add liquid soap to the water and stir it. We find that the needle sinks.
![]()
![]()
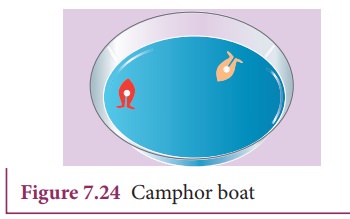
Activity 2:
Take
a plastic sheet and cut out a piece in the shape of a boat (Figure 7.24). A
tapering and smooth front with a notch at the back is suggested. Put a piece of
camphor into the notch of the boat. Gently release the boat on the surface of
the water and we find that the boat is propelled forward when the camphor
dissolves. The surface tension is lowered, as the camphor dissolves and
produces a difference in surface tension in the water nearby the notch. This
causes the water to flow away from the back of the boat, which moves the boat
forward.
Related Topics