Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Summary: Chemistry Periodic Classification of Elements
SUMMARY
The periodic table was developed to systematically arrange
the different elements. Lavoisier made the first attempt to arrange the known
elements in a particular order based on properties. This followed by Johann
Dobereiner, A. E. B. de Chancourtois and Newlands. First meaningful periodic
table was constructed by Mendeleeve based on atomic mass. This was later
modified based on the modern periodic law which states that the properties of
elements are the periodic functions of their atomic numbers. The modern
periodic table is made up of 18 groups and 7 periods.
The elements in the same groups have similar properties
because their valence shell electronic configurations are similar. The
properties of the elements of the same period differ because they have
different valence shell electronic configurations. On the basis of electronic
configuration the elements are also classified as s-block, p-block, d-block and
f-block elements. The elements belonging to “s, p, d and f ” blocks have unique
characteristic
In this table, more than 78% of all known
elements are metals. They appear on the left side of the periodic table.
Non-metals are located at the top right hand side of the periodic table. Some
elements show properties that are characteristic of both metals and non-metals
and are called semi-metals or metalloids.
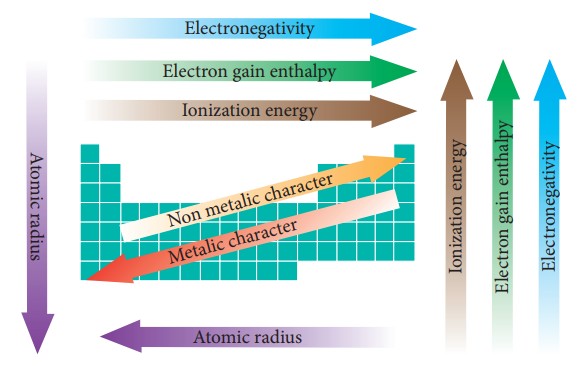
The periodic properties such as atom-ic radius, ionic
radius, ionization enthalpy, electron gain enthalpy, electronegativity are
possessing periodic trends. The variations of these properties are described in
the follow-ing scheme.
The elements at the extreme left exhibit strong reducing
property whereas the elements at extreme right strong oxidizing property. The
reactivity of elements at the centre of the periodic table is low compared to
elements at the extreme right and left. The similarity in chemical properties
observed between the elements of second and third period which are diagonally related.
Related Topics