Science - Sublimation | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Sublimation
Sublimation
Have you noticed that the moth balls which we place
in our cloth cupboards disappear after a few days? But you may still get the
smell of those naphthalene balls even after they ‘disappear’. What has
happened?
Certain solids change directly to gas without
passing through the liquid state. The direct change of a state from solid to
gas is called sublimation. On cooling these vapours come back to its original
(or) actual state.
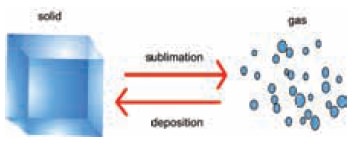
For example, dry ice (frozen CO2),
naphthalene, ammonium chloride and iodine sublime. The energy required for this
change of state can be derived either from the surrounding or from the heat
supplied. Inverse of this process is called deposition, in which gas particles
lose heat and change their phase to solid.
Related Topics