Chapter: Medical Physiology: Respiratory Insufficiency-Pathophysiology, Diagnosis, Oxygen Therapy
Study of Blood Gases and Blood pH
Study of Blood Gases and Blood pH
Among the most fundamental of all tests of pulmonary performance are deter-minations of the blood PO2, CO2, and pH. It is often important to make these measurements rapidly as an aid in determining appropriate therapy for acute respiratory distress or acute abnormalities of acid-base balance. Several simple and rapid methods have been developed to make these measurements within minutes, using no more than a few drops of blood. They are the following.
Determination of Blood pH. Blood pH is measured using a glass pH electrode ofthe type used in all chemical laboratories. However, the electrodes used for this purpose are miniaturized. The voltage generated by the glass electrode is a direct measure of pH, and this is generally read directly from a voltmeter scale, or it is recorded on a chart.
Determination of Blood CO2. A glass electrode pH meter can also be used to deter-mine blood CO2 in the following way: When a weak solution of sodium bicar-bonate is exposed to carbon dioxide gas, the carbon dioxide dissolves in the solution until an equilibrium state is established. In this equilibrium state, the pH of the solution is a function of the carbon dioxide and bicarbonate ion con-centrations in accordance with the Henderson-Hasselbalch equation.; that is,
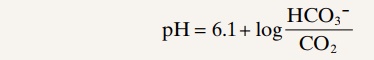
When the glass electrode is used to measure CO2 in blood, a miniature glass electrode is surrounded by a thin plastic membrane. In the space between the electrode and plastic membrane is a solution of sodium bicarbonate of known concentration. Blood is then superfused onto the outer surface of the plastic membrane, allowing carbon dioxide to diffuse from the blood into the bicarbonate solution. Only a drop or so of blood is required. Next, the pH is measured by the glass electrode, and the CO2 is calculated by use of the above formula.
Determination of Blood PO2. The concentration of oxygenin a fluid can be measured by a technique called polarography. Electric current is made toflowbetween a small negative electrode and the solution. If the voltage of the electrode is more than -0.6 volt different from the voltage of the solution, oxygen will deposit on the electrode. Furthermore, the rate of current flow through the electrode will be directly pro-portional to the concentration of oxygen (and there-fore to PO2 as well). In practice, a negative platinum electrode with a surface area of about 1 square mil-limeter is used, and this is separated from the blood by a thin plastic membrane that allows diffusion of oxygen but not diffusion of proteins or other sub-stances that will “poison” the electrode.
Often all three of the measuring devices for pH, CO2, and Po2 are built into the same apparatus, and all these measurements can be made within a minute or so using a single, droplet-size sample of blood. Thus, changes in the blood gases and pH can be followed almost moment by moment at the bedside.
Related Topics