Chapter: Biochemistry: Carbohydrates
Structures and Functions of Polysaccharides
Structures and Functions of
Polysaccharides
When many monosaccharides are linked together, the result is a polysaccharide. Polysaccharides that
occur in organisms are usually composed of a very few types of monosaccharide
components. A polymer that consists of only one type of monosaccharide is a homopolysaccharide; a polymer that
consists of more than one type of monosaccharide is a heteropolysaccharide. Glucose is the most common monomer. When
there is more than one type of monomer, frequently only two types of molecules
occur in a repeating sequence. A complete characterization of a polysaccharide
includes specification of which monomers are present and, if necessary, the
sequence of monomers. It also requires that the type of glycosidic linkage be
specified. We shall see the importance of the type of glycosidic linkage as we
discuss different polysaccharides, since the nature of the linkage determines
function. Cellulose and chitin are polysaccharides with β-glycosidic linkages, and
both are structural materials. Starch and glycogen,also polysaccharides, have α-glycosidic linkages, and
they serve as carbohydrate-storage polymers in plants and animals,
respectively.
How do cellulose and starch differ from one another?
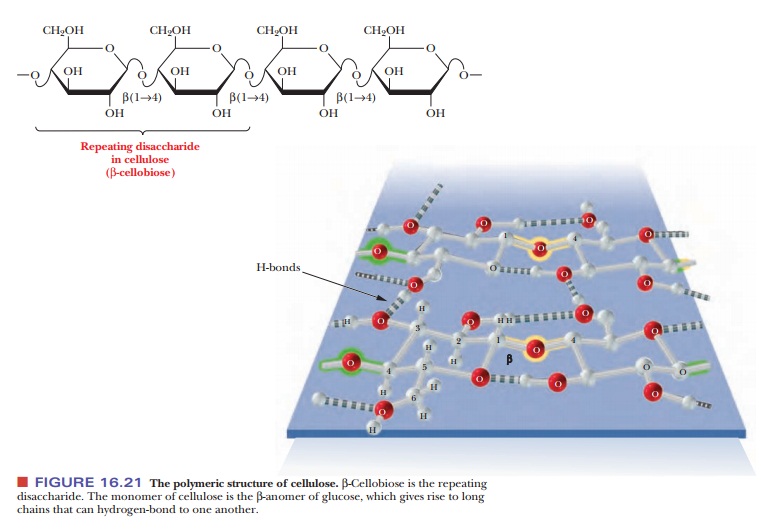
Cellulose is the major structural component of plants, especially of wood and plant fibers. It is a linear homopolysaccharide of β-D-glucose, and all residues are linked in β(1 - > 4) glycosidic bonds (Figure 16.21). Individual polysaccharide chains are hydrogen-bonded together, giving plant fibers their mechanical strength. Animals lack the enzymes, called cellulases, that hydrolyze cellulose to glucose. Such enzymes attack the β-linkages between glucoses, which is common to structural polymers; the α-linkage between glucoses, which animals can digest, is characteristic of energy-storage polymers such as starch (Figure 16.22). Cellulases are found in certain bacteria, including the bacteria that inhabit the digestive tracts of insects, such as termites, and grazing animals, such as cattle and horses. The presence of these bacteria explains why cows and horses can live on grass and hay but humans cannot. The damage done by termites to the wooden parts of buildings arises from their ability to use cellulose in wood as a nutrient—owing to the presence of suitable bacteria in their digestive tracts.
Is there more than one form of starch?
The importance of carbohydrates as energy sources suggests that
there is some use for polysaccharides in metabolism. We shall now discuss in
more detail some polysaccharides, such as starches, that serve as vehicles for
storage of glucose.
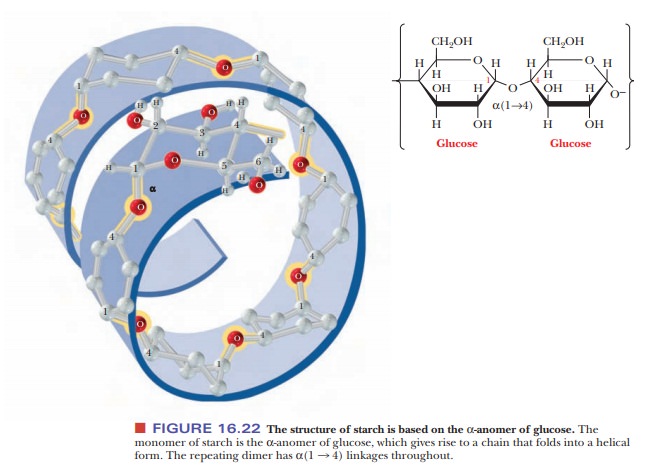
Starches are
polymers ofα-D-glucose that occur in plant cells, usually as starchgranules in
the cytosol. Note that there is anα-linkage in starch, in contrast with the β-linkage in cellulose. The
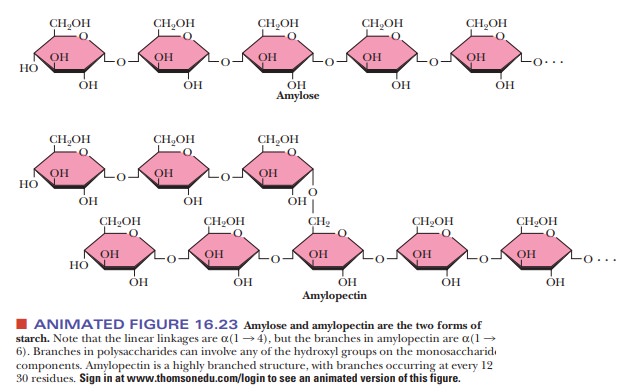
types of starches can be distinguished from one another by their degrees of
chain branching. Amylose is a linear polymer of glu-cose, with all the residues
linked together by α(1 -
> 4) bonds. Amylopectin is a branched chain polymer, with the branches
starting at α(1 -
> 6) linkages along the chain of α(1 - > 4) linkages (Figure 16.23). The most
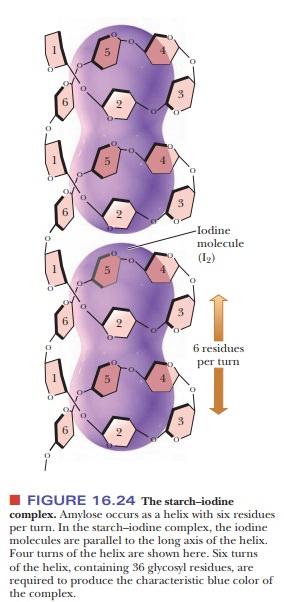
usual conformation of amylose is a helix with six residues per turn. Iodine
molecules can fit inside the helix to form a starch–iodine complex, which has a
characteristic dark-blue color (Figure 16.24). The formation of this complex is
a well-known test for the pres-ence of starch. If there is a preferred
conformation for amylopectin, it is not yet known. (It is known that the color of the product obtained when amylopectin
and glycogen react with iodine is red-brown, not blue.)

Because starches are storage molecules, there must be a mechanism
for releasing glucose from starch when the organism needs energy. Both plants
and animals contain enzymes that hydrolyze starches. Two of these enzymes,
known as α- and β-amylase (the α and β do not signify anomeric forms in this case),
attack α(1 -
> 4) linkages. β-amylase
is an exoglycosidase that cleaves
from the nonreducing end of the polymer. Maltose, a dimer of glucose, is the
prod-uct of reaction. The other enzyme, α-amylase, is an endoglycosidase, which can hydrolyze a glycosidic linkage anywhere
along the chain to produce glucose and maltose. Amylose can be completely
degraded to glucose and maltose by the two amylases, but amylopectin is not
completely degraded because the
However, debranching enzymes occur in both plants and animals; they degrade
the α(1 -
> 6) linkages. When these enzymes are combined with the amylases, they
contribute to the complete deg-radation of both forms of starch.
How is glycogen related to starch?
Although starches occur only in plants, there is a similar
carbohydrate storage polymer in animals. Glycogen
is a branched-chain polymer of α-D-glucose, and in this respect
it is similar to the amylopectin fraction of starch. Like amylopectin, glycogen
consists of a chain of α(1 - > 4) linkages with α(1 - > 6) linkages at the
branch points. The main difference between glycogen and amylopectin is that
glycogen is more highly branched (Figure 16.25). Branch points occur about
every 10 residues in glycogen and about every 25 residues in amylopectin. In
glycogen, the average chain length is 13 glucose residues, and there are 12
layers of branching. At the heart of every glycogen molecule is a protein
called glycogenin. Glycogen is found in animal cells in granules similar to the
starch granules in plant cells. Glycogen granules are observed in well-fed
liver and muscle cells, but they are not seen in some other cell types, such as
brain and heart cells under normal conditions. Some athletes, particularly
long-distance runners, try to build up their glycogen reserves before a race by
eating large amounts of carbohydrates. When the organism needs energy, various
degradative enzymes remove glucose units. Glycogen phosphorylase is one such
enzyme; it cleaves one glucose at a time from the nonreducing end of a branch
to produce glucose-1-phosphate, which then enters the metabolic pathways of
carbohydrate breakdown. Debranching enzymes also play a role in the complete
breakdown of glycogen. The number of branch points is significant for two
reasons. First, a more branched polysaccharide is more water soluble. This may
not be as important for a plant, but, for a mammal, the amount of glycogen in
solution is. There are glycogen-storage diseases caused by lower-than-normal
levels of branching enzymes. The glycogen products resemble starch and can fall
out of solution, forming glycogen crystals in the muscles and liver.

Second, when an organism needs energy quickly, the glycogen phosphorylase has more potential targets if there are more branches, allowing a quicker mobilization of glucose. Again, this is not as important to a plant, so there was no evolutionary pressure to make starch highly branched.
What is chitin?
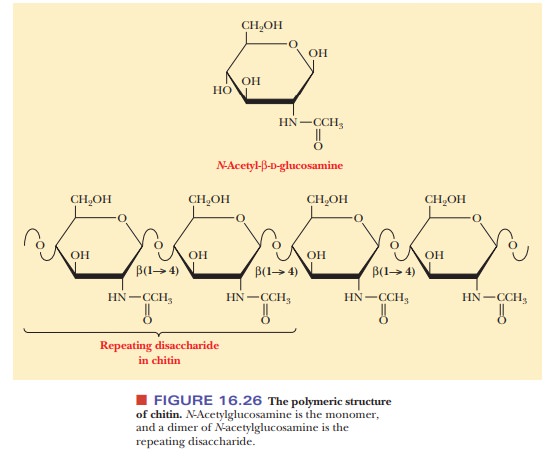
A polysaccharide that is similar to cellulose in both structure and
function is chitin, which is also a linear homopolysaccharide with all the
residues linked in β(1 -
> 4) glycosidic bonds. Chitin differs from cellulose in the nature of the
monosaccharide unit; in cellulose, the monomer is β-D-glucose; in chitin, the
monomer is N-acetyl-β-D-glucosamine.
The latter compound differs from glucose only in the substitution of the N-acetylamino group (—NH—CO—CH3) for the hydroxyl group (—OH) on
carbon C-2 (Figure 16.26). Like cellulose, chitin individual strands are held
together by hydrogen bonds. It is a major structural component of the
exoskeletons of invertebrates such as insects and crustaceans (a group that
includes lobsters and shrimp), and it also occurs in cell walls of algae,
fungi, and yeasts.
What role do polysaccharides play in the structure of cell walls?
In organisms that have cell walls, such as bacteria and plants, the
walls consist largely of polysaccharides. The cell walls of bacteria and plants
have biochemical differences, however.
Heteropolysaccharides are major components of bacterial cell walls. A distin-guishing feature of prokaryotic cell
walls is that the polysaccharides are cross-linked by peptides. The repeating
unit of the polysaccharide consists of two residues held together by β(1 - > 4) glycosidic links,
as was the case in cellulose and chitin. One of the two monomers is N-acetyl-D-glucosamine,
which occurs in chitin, and the other monomer is N-acetylmuramic acid (Figure 16.27a). The structure of N-acetylmuramic acid differs from that
of N-acetylglucosamine by the
substitution of a lactic acid side chain [—O—CH(CH3)—COOH]
for the hydroxyl group (—OH) on carbon 3. N-Acetylmuramic
acid is found only in prokaryotic cell walls; it does not occur in eukaryotic
cell walls.
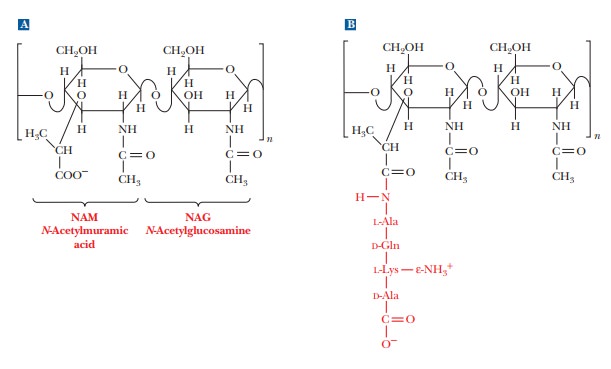
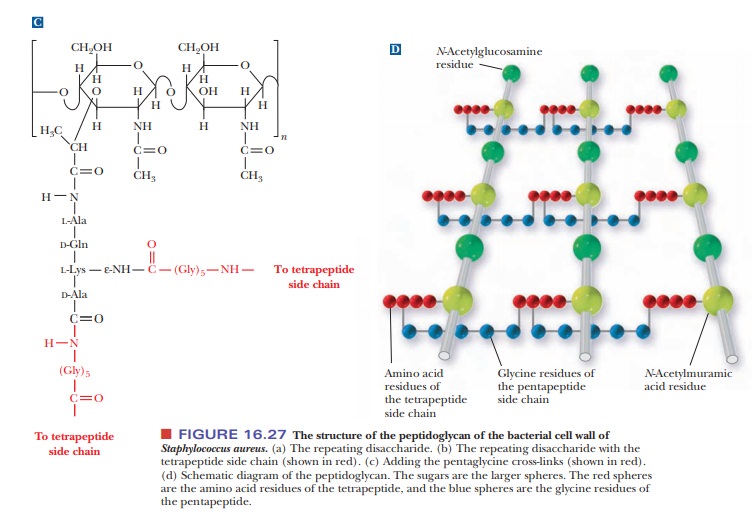
The cross-links in bacterial cell walls consist of small peptides.
We shall use one of the best-known examples as an illustration. In the cell
wall of the bacterium Staphylococcus
aureus, an oligomer of four amino acids (a tetramer) is bonded to N-acetylmuramic acid, forming a side
chain (Figure 16.27b). The tetrapeptidesare themselves cross-linked by another
small peptide, in this case consisting of five amino acids.
The carboxyl group of the lactic acid side chain of N-acetylmuramic acid forms an amide bond
with the N-terminal end of a tetrapeptide that has the sequence L-Ala-D-Gln-L-Lys-D-Ala.
Recall that bacterial cell walls are one of the few places where D-amino acids occur in nature. The occurrence of D-amino acids andN-acetylmu-ramic
acid in bacterial cell walls but not in plant cell walls shows a biochemical as
well as structural difference between prokaryotes and eukaryotes.
The tetrapeptide forms two cross-links, both of them to a
pentapeptide that consists of five glycine residues, (Gly)5. The
glycine pentamers form peptide bonds to the C-terminal end and to the
side-chain ε-amino
group of the lysine in the tetrapeptide [Figure 16.27(c)]. This extensive
cross-linking produces a three-dimensional network of considerable mechanical
strength, which is why bacterial cell walls are extremely difficult to disrupt.
The material that results from the cross-linking of polysaccharides by peptides
is a peptidoglycan [Figure
16.25(d)], so named because it has both peptide and carbohydrate components.
Plant cell walls consist
largely ofcellulose.The other
important polysaccha-ride component found in plant cell walls is pectin, a polymer made up mostly of D-galacturonic acid, a derivative of galactose in which the hydroxyl
group on carbon C-6 has been oxidized to a carboxyl group.
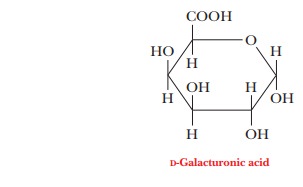
Pectin is extracted from plants because it has commercial
importance in the food-processing industry as a gelling agent in yogurt, fruit
preserves, jams, and jellies. The major nonpolysaccharide component in plant
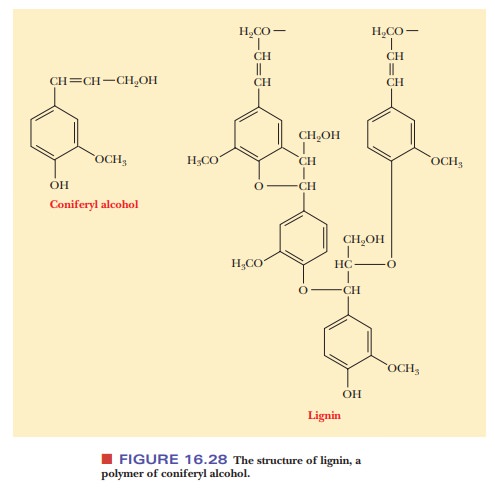
cell walls, especially in woody plants, is lignin
(Latin lignum, “wood”). Lignin is a
polymer of coniferyl alcohol, and it is a very tough and durable material
(Figure 16.28). Unlike bacterial cell walls, plant cell walls contain
comparatively little peptide or protein.
Do polysaccharides play any specific roles in connective tissue?
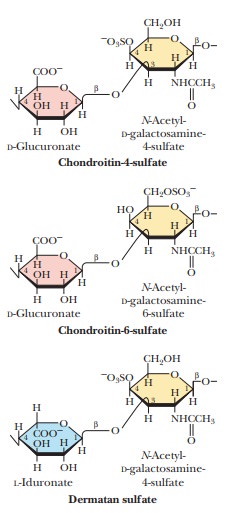
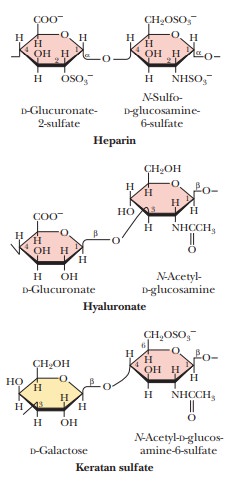
Glycosaminoglycans are a
type of polysaccharide based on a repeating disaccharidein which one of the
sugars is an amino sugar and at least one of them has a negative charge owing
to the presence of a sulfate group or a carboxyl group. These polysaccharides
are involved in a wide variety of cellular functions and tissues. Figure 16.29
shows the disaccharide structure of the most common ones. Heparin is a natural
anticoagulant that helps prevent blood clots. Hyaluronic acid is a component of
the vitreous humor of the eye and of the lubricating fluid of joints. The
chondroitin sulfates and keratan sulfate are components of connective tissue.
Glucosamine sulfate and chondroitin sulfate are sold in large quantities as
over-the-counter drugs used to help repair frayed or otherwise damaged
cartilage, especially in knees. Many people who are advised that they need knee
surgery for damaged ligaments look for improvement first with a two- or
three-month regimen of these glycosaminoglycans. Questions exist about the
efficacy of this treatment, so it will be interesting to see what future it may
have.
Summary
Polysaccharides are formed by linking monomeric sugars through
glyco-sidic linkages.
Starch and glycogen are energy-storage polymers of sugars.
Cellulose and chitin are structural polymers.
Polysaccharides are important components of cell walls in bacteria
and plants.
Related Topics