Chapter: Modern Analytical Chemistry: Basic Tools of Analytical Chemistry
Stoichiometric Calculations
Stoichiometric
Calculations
A balanced chemical
reaction indicates the quantitative relationships between the moles of reactants and
products. These stoichiometric relationships provide the basis for many analytical calculations. Consider, for example, the problem of deter-
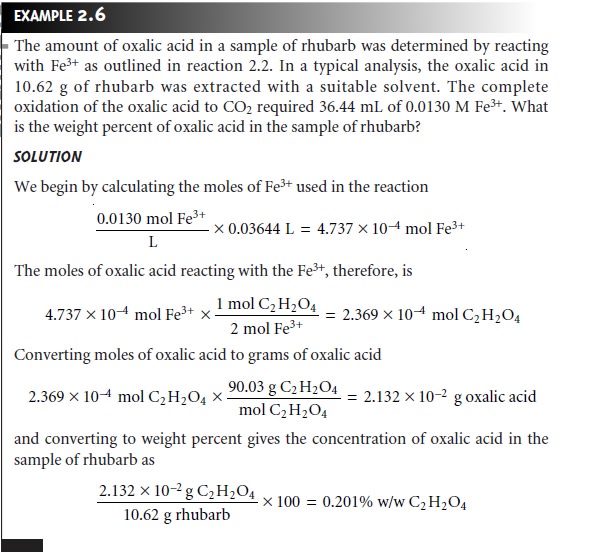
mining the amount of oxalic
acid, H2C2O4, in rhubarb. One
method for this
analy- sis uses the following reaction
in which we oxidize oxalic
acid to CO2.
2Fe3+(aq) + H2C2O4(aq) + 2H2O(l) →
2Fe2+(aq) + 2CO2(g) + 2H3O+(aq)
….2.2
The balanced chemical reaction
provides the stoichiometric relationship between the moles of Fe3+ used and the moles
of oxalic acid in the sample being
analyzed— specifically, one mole
of oxalic acid
reacts with two
moles of Fe3+.
As shown in Example 2.6, the balanced chemical
reaction can be used to determine the amount of oxalic
acid in a sample, provided that information about
the number of moles of Fe3+ is
known.
In the analysis
described in Example
2.6 oxalic acid already was present in the
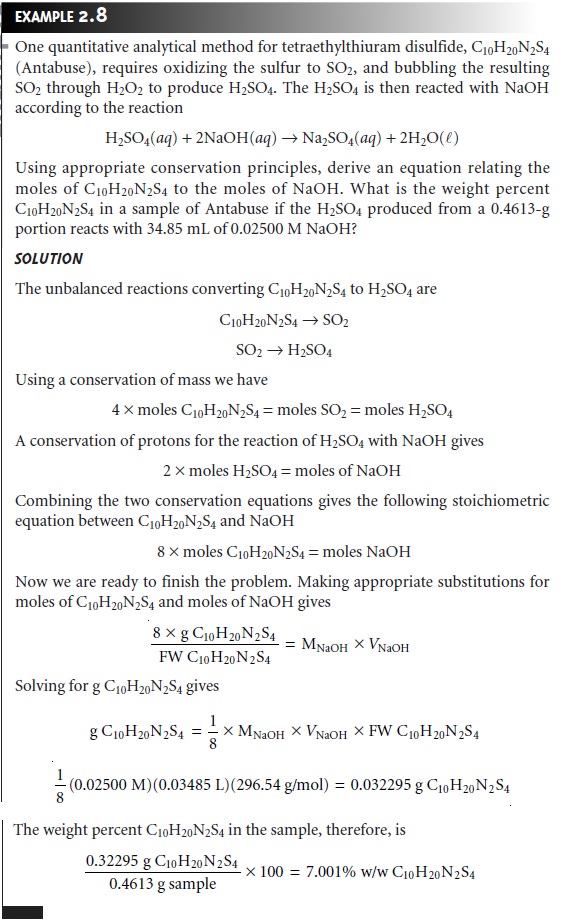
desired form. In many analytical methods the compound
to be determined must be converted to another form prior to analysis. For example, one method for the quan- titative analysis of tetraethylthiuram disulfide (C10H20N2S4), the
active ingredient in the
drug Antabuse (disulfiram), requires oxidizing the S to SO2, bubbling
the SO2 through H2O2 to produce H2SO4, followed by an acid–base titration of the
H2SO4 with NaOH. Although
we can write and balance
chemical reactions for each of these
steps, it often is easier
to apply the
principle of the
conservation of reaction units.
A reaction unit is that part of a chemical
species involved in a reaction. Con- sider, for example, the general unbalanced chemical reaction
A + B → Products
Conservation of reaction units requires that
the number of reaction units
associated with the reactant
A equal the number of reaction units associated with the reactant
B. Translating the previous
statement into mathematical form gives
Number of reaction units per A x moles A= number of reaction units per B x moles B ……………….. 2.3
If
we know the
moles of A and the
number of reaction units associated with
A and B, then
we can calculate the moles of B. Note
that a conservation of reaction units, as defined by equation
2.3, can only be applied
between two species.
There are five important principles involving a conservation of reaction units: mass, charge,
pro- tons, electron pairs,
and electrons.
Conservation of Mass
The easiest principle to appreciate is conservation of mass. Except
for nuclear reac- tions, an element’s total
mass at the end of a reaction
must be the same as that pres- ent at the beginning of the reaction; thus, an element
serves as the
most fundamen- tal reaction
unit. Consider, for example, the combustion of butane to produce CO2 and H2O, for which
the unbalanced reaction
is
C4H10(g) + O2(g) → CO2(g) +
H2O(g)
All the carbon
in CO2 comes
from the butane,
thus we can select carbon
as a reac- tion unit. Since
there are four
carbon atoms in butane, and
one carbon atom
in CO2, we write
4 x moles C4H10
= 1 x moles CO2
Hydrogen also can be selected
as a reaction unit since
all the hydrogen
in butane ends up in the
H2O produced during
combustion. Thus, we can write
10 x moles C4H10
= 2 x moles H2O
Hydrogen also can be selected
as a reaction unit since
all the hydrogen
in butane ends up in the
H2O produced during
combustion. Thus, we can write
10 x moles C4H10
= 2 x moles H2O
Although the mass of oxygen
is conserved during
the reaction, we cannot apply equation 2.3 because the O2 used
during combustion does not end up in a single product.
Conservation of mass also can, with care, be applied to groups
of atoms. For example, the ammonium ion, NH4+, can be precipitated as
Fe(NH4) (SO4) . 6H2O.
Selecting NH4+ as the reaction unit gives
2 x moles Fe(NH4)2(SO4)2
· 6H2O = 1 x moles NH4+
Conservation of Charge
The stoichiometry between
two reactants in a precipitation reaction is governed
by a conservation of charge, requiring
that the total cation charge and the total anion charge in the precipitate be equal. The
reaction units in a precipitation reaction, therefore, are the absolute values
of the charges on the cation and anion that make
up the precipitate. Applying equation
2.3 to a precipitate of Ca3(PO4)2 formed
from the reaction of Ca2+ and PO43–, we write
2 x moles Ca2+ = 3 x moles PO43–
Conservation of Protons
In an acid–base reaction, the reaction
unit is the proton. For an acid, the num- ber
of reaction units is given by the number of protons that can be donated
to the base; and for a base, the number of reaction
units is the number of protons
that the base can accept from the acid. In the reaction
between H3PO4 and NaOH, for example,
the weak acid H3PO4 can donate all three of its pro-
tons to NaOH, whereas the strong base NaOH can accept one proton. Thus, we
write
3 x moles H3PO4 = 1 x moles NaOH
Care must be exercised in determining the number of reaction units
associ- ated with the acid and base. The number of reaction units
for an acid, for in- stance, depends
not on how many acidic
protons are present,
but on how many of the protons are capable of reacting with the chosen base. In the reaction
be- tween H3PO4 and NH3
H3PO4(aq) + 2NH3(aq)
< = = = = > HPO4–(aq) + 2NH4+(aq)
a conservation of protons requires that
2 x moles H3PO4 = moles of NH3
Conservation of Electron Pairs
In a complexation reaction, the reaction
unit is an electron pair.
For the metal,
the number of reaction
units is the number of coordination sites available for binding
ligands. For the ligand, the number of reaction units
is equivalent to the number
of electron pairs that can be donated to the metal. One of the most important analyti- cal complexation reactions is that between
the ligand ethylenediaminetetracetic acid (EDTA), which can donate
6 electron pairs
and 6 coordinate metal ions,
such as Cu2+; thus
6 x mole Cu2+ = 6 x moles EDTA
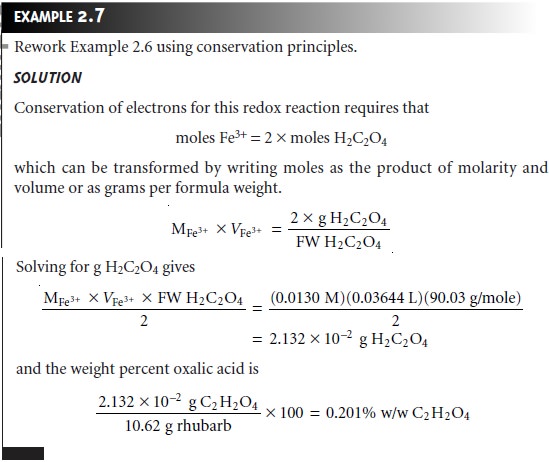
Conservation of Electrons
In a redox
reaction, the reaction
unit is an electron transferred from a reducing agent to an oxidizing agent. The number
of reaction units
for a reducing agent is equal to the number
of electrons released
during its oxidation. For an oxidizing agent, the number of reaction units
is given by the number
of electrons needed
to cause its reduction. In the reaction
between Fe3+ and oxalic acid (reaction 2.2), for example, Fe3+ undergoes a 1-electron reduction. Each carbon atom in oxalic
acid is initially present
in a +3 oxidation state,
whereas the carbon
atom in CO2 is in a +4
oxidation state. Thus,
we can write
1 x moles Fe3+ = 2 x moles of H2C2O4
Note that the moles of oxalic acid are multiplied by 2 since
there are two carbon
atoms, each of which undergoes a 1-electron oxidation.
Using Conservation Principles in Stoichiometry Problems
As shown in the following
examples, the application of conservation principles sim- plifies stoichiometric
calculations.
Related Topics