Chapter: Biochemistry: Carbohydrates
Some Important Oligosaccharides
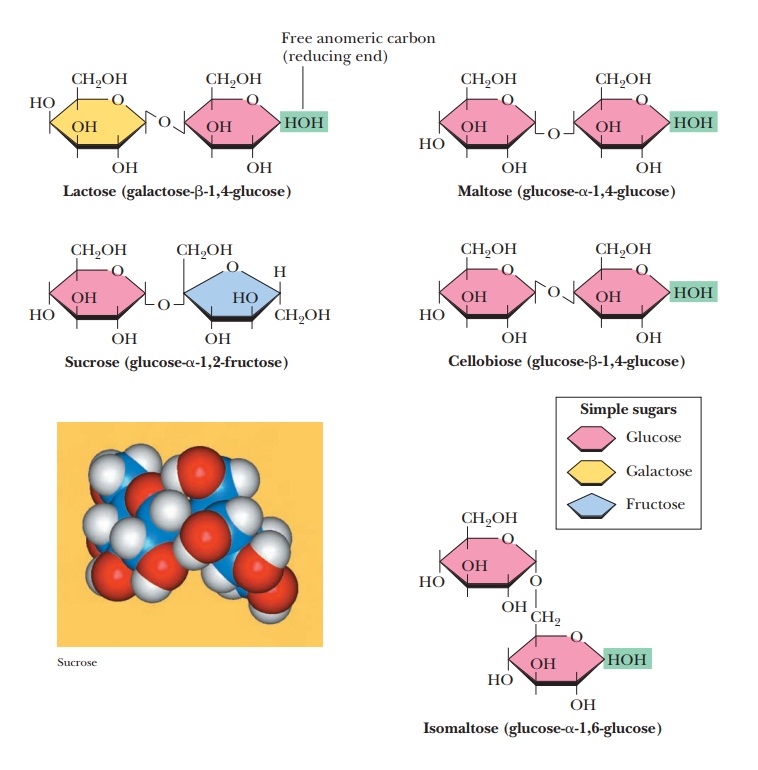
Some Important Oligosaccharides
Oligomers of sugars frequently occur as disaccharides, formed by linking two monosaccharide units by
glycosidic bonds. Three of the most important examples of oligosaccharides are
disaccharides. They are sucrose, lactose, and maltose (Figure 16.19). Two other
disaccharides, isomaltose and cellobiose, are shown for comparison.
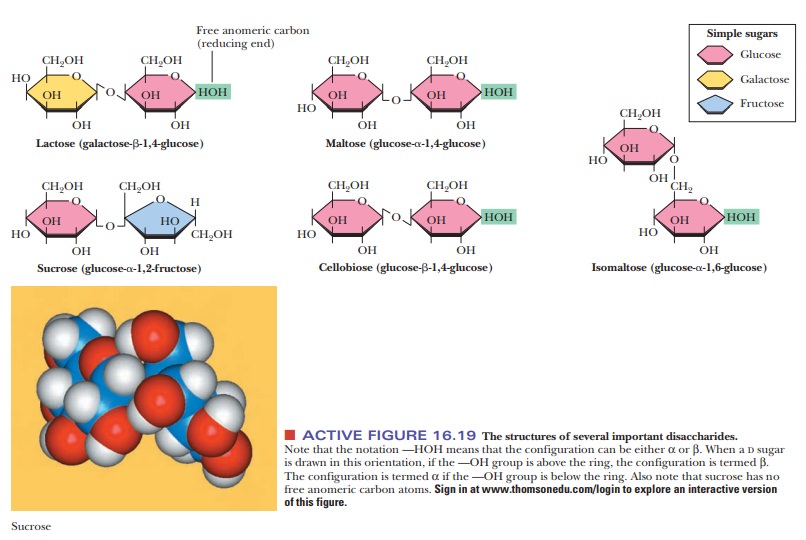
What makes sucrose an important compound?
Sucrose is
common table sugar, which is extracted from sugarcane and sugarbeets. The
monosaccharide units that make up sucrose are α-D-glucose and β-D-fructose.
Glucose (an aldohexose) is a pyranose, and fructose (a ketohexose) is a
furanose. The α C-1
carbon of the glucose is linked to the β C-2 carbon of the fructose (Figure 16.19) in a
glycosidic linkage that has the notation α,β(1 - > 2). Sucrose is not a reducing sugar
because both anomeric groups are involved in the glycosidic linkage. Free
glucose is a reducing sugar, and free fructose can also give a positive test,
even though it is a ketone rather than an aldehyde in the open-chain form.
Fructose and ketoses in general can act as reducing sugars because they can
isomerize to aldoses in a rather complex rearrangement reaction. (We need not
concern ourselves with the details of this isomerization.)
When animals consume sucrose, it is hydrolyzed to glucose and fructose, which are then degraded by metabolic processes to provide energy. Humans consume large quantities of sucrose, and excess consumption can contribute to health problems; this fact has led to a search for other sweetening agents.
One that has been proposed is fructose itself. It is sweeter than
sucrose; therefore, a smaller amount (by weight) of fructose than sucrose can
produce the same sweetening effect with fewer calories. Consequently,
high-fructose corn syrup is frequently used in food processing. The presence of
fructose changes the texture of food, and the reaction to the change tends to
depend on the preference of the consumer. Artificial sweeteners have been
produced in the laboratory and have frequently been suspected of having harmful
side effects; the ensuing controversies bear eloquent testimony to the human
craving for sweets. Saccharin, for example, has been found to cause cancer in
laboratory animals, as have cyclamates, but the applicability of these results
to human carcinogenesis has been questioned by some. Aspartame has been
suspected of causing neu-rological problems, especially in individuals whose
metabolisms cannot tolerate phenylalanine.
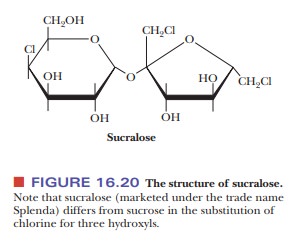
Another artificial sweetener is a derivative of sucrose. This
substance, sucra-lose, which is marketed under the trade name Splenda, differs
from sucrose in two ways (Figure 16.20). The first difference is that three of
the hydroxyl groups have been replaced with three chlorine atoms. The second is
that the configuration at carbon atom 4 of the six-membered pyranose ring of
glucose has been inverted, producing a galactose derivative. The three hydroxyl
groups that have been replaced by chlorine atoms are those bonded to carbon
atoms 1 and 6 of the fructose moiety and to carbon atom 4 of the galactose
moiety. Sucralose is not metabolized by the body, and, consequently, it does
not pro-vide calories. Tests conducted so far, as well as anecdotal evidence,
indicate that it is a safe sugar substitute. It is likely to find wide use in
the near future. We can safely predict that the search for nonfattening
sweeteners will continue and that it will be accompanied by controversy.
Are any other disaccharides important to us?
Lactose is a disaccharidemade up of β-D-galactose
and D-glucose. Galactose is the C-4 epimer of
glucose. In other words, the only difference between glucose and galactose is
inversion of configuration at C-4. The glycosidic linkage is β(1 - > 4), between the
anomeric carbon C-1 of the β form of galactose and the C-4 carbon of
glucose (Figure 16.19). Since the anomeric carbon of glucose is not involved in
the glycosidic linkage, it can be in either the α or the β form. The two anomeric forms of lactose can be
specified, and the designation refers to the glucose residue; galactose must be
present as the β-anomer,
since the β form of
galactose is required by the structure of lactose. Lactose is a reducing sugar
because the group at the anomeric carbon of the glucose portion is not involved
in a glycosidic linkage, so it is free to react with oxidizing agents.
Maltose is a
disaccharide obtained from the hydrolysis of starch. It consistsof two residues
of D-glucose in an α(1 - > 4) linkage. Maltose differs from cel-lobiose, a disaccharide that is
obtained from the hydrolysis of cellulose, only inthe glycosidic linkage. In
cellobiose, the two residues of D-glucose are bonded together
in a β(1 -
> 4) linkage (Figure 16.19). Mammals can digest maltose, but not cellobiose.
Yeast, specifically brewer’s yeast, contains enzymes that hydrolyze the starch
in sprouted barley (barley malt) first to maltose and then to glucose, which is
fermented in the brewing of beer. Maltose is also used in other beverages, such
as malted milk.
Summary
The disaccharide sucrose is common table sugar. It consists of
glucose and fructose linked by a glycosidic bond.
Lactose, found in milk, and maltose, obtained from starch, are two
other common disaccharides.
Related Topics