Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Selection of pH indicators and Titration
pH INDICATORS
An indicator is a substance which indicates the
completion of a reaction by sharp colour change at the end point without taking
part in the reaction. The substances which are used to indicate the end point
in acid-base reactions are called as acid-base indicators. (e.g.,)
phenolphthalein and methyl orange.
Those substances which change to specific
colours in different pH range values of the medium are called as pH indicators.
Incidentally pH indicators are used as acid-base indicators also.
Selection of pH indicators
Every pH indicator changes its colour
specifically in a ranging pH which is called as indicator range. For some of
the indicators, the indicator ranges are given as below.
Colour of the indicator
Indicator pH range Acidic solution Basic solution
Methyl
orange : 3.1 - 4.4 Pink Yellow
Methyl
red : 4.4 - 6.2 Red Yellow
Phenol
red : 6.8 - 8.4 Yellow Red
Phenolphthalein
: 8.3 - 10 Colourless Pink
When a base is added to a solution of an acid,
the H+ ions will be slowly neutalised by the OH- ions of
the base. Hence, there is a steady decrease in the H+ ion
concentration and pH value increases uniformly. At the end point there is a
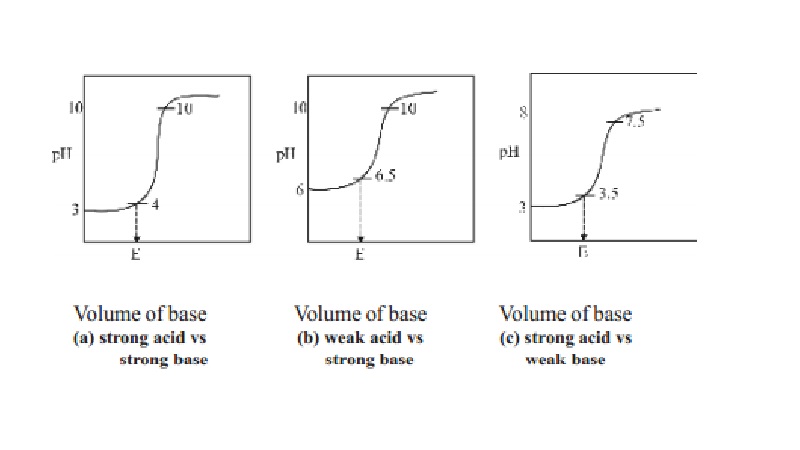
steep rise in the pH value. The pH values can be plotted against the volume of
the base added and the curve so obtained is called titration curve. The
titration curves are useful in the choice of a suitable indicator in an
acid-base titration. A suitable indicator in an acid-base titration is one
whose range is well within the sharp rising portion of the titration curve.
Thus the choice of a suitable indicator for any titration depends on the nature
of the acid and base involved and the working range of the indicator.
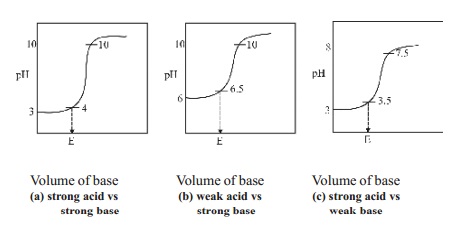
1.Titration of a strong acid against a strong
base : (Example, HCl vs NaOH)
In this type of titration, the change in the pH
value at the end point is roughly from 4 to 10. Therefore any indicator which
changes its colour within this range may be used as a suitable indicator in the
titration of strong acid against strong base and phenolphthalein can be used as
indicators for this type of titrations.
2.Titration of a weak acid against a strong
base : (Example, Oxalic acid vs NaOH)
There is a little change in the pH value at the
end point in this type of titration. The pH value changes from 6.5 to 10. Thus
phenolphthalein is the suitable indicator for this titration as its working
range is 8.3 - 10. Methyl orange is not a suitable indicator. Since it has a
working range below pH 5.
3.Titration of strong acid against weak base :
(Example, HCl vs Na2CO3]
When a strong acid like HCl is titrated against
a weak base like Na2CO3, the pH changes from 3.5 to 7.5
at the end point. The best indicator for this type of titration is methyl
orange which changes its colour within this pH range.
4.Titration of weak acid against weak base : (Example,
CH3COOH vs NH4OH)
In this sypte of titration there is no sharp
change in the pH value at the end point. Therefore, in the titration of a weak
acid against a weak base none of the indicators shown in the table are quite
satisfactory.
There are two theories to explain the function
of acid-base indicators.
1. Ostwald's theory
This theory was proposed by Ostwald's in 1891.
It is based on Arrhenius theory. According to this theory, the acid-base
indicator is either a weak acid or a weak base. They are partially ionised in
solution. The ionised and unionised forms have different colours. The indicator
exists predominantly in one of the two forms depending on the nature of the
medium and hence there is colour change when the nature of the medium changes.
Phenolphthalein is a weak acid and it is partially ionised in solutions.
HPh (Unionised form (colourless) < -- -- -- > H+ + Ph -
(ionised form (pink) )
In acidic medium, excess H+ ions are
present which suppress the dissociation of HpH due to common ion effect. Hence
the indicator exists predominantly in unionised form and it is colourless. In
alkaline medium, the OH- ion neutralises H+ ion to form
water. Consequently the dissociation of HpH is favoured and the indicator is
predominantly in the ionised form and it is pink in colour.
Methyl orange is a weak base and its ionisation
can be written as
MeOH (Unionised form (yellow)) < -- -- -- > Me+ + OH- (ionised form (pink))
In the presence of a base excess OH-
ions suppress the dissociation of MeOH due to common ion effect. Hence in basic
medium, the indicator is mostly in unionised form which is yellow.
In acidic solution the H+ ions
combine with OH- ions to form unionised water. Hence in acidic
solution, the indicator is mostly in ionised form and has pink colour.
This theory also explains why phenolphthalein
is not a suitable indicator in the titration of a strong acid against a weak
base. The reason is the OH- ions produced by the weak base at the
end point is too low to cause the ionisation of phenolphthalein. Hence, the
pink colour does not appear exactly at the equivalence point. The pink colour
appears only after a sufficient excess of the weak base is added.
For a similar reason, methyl orange is not a
suitable indicator in the titration of a strong base against a weak acid. The
weak acid does not furnish sufficient H+ ions to shift the
equilibrium towards the right. A sufficient excess of the weak acid has to be
added to get the colour change.
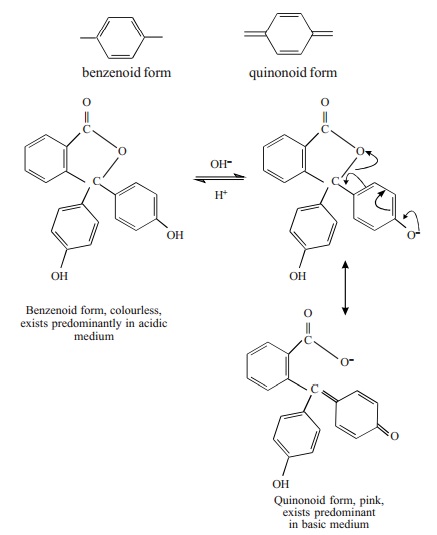
Quinonoid Theory
According to this theory the colour change of
an acid-base indicator arises as a result of structural change. It is supposed
that an indicator exists as an equilibrium mixture of two tautomeric forms
namely, benzenoid and quinonoid forms.
One form exists in acidic solution and the
other form in basic solution. At least one of the tautomers is a weak acid or a
weak base. The two forms possess two different colours and as the pH of the
solution containing the indicator is changed, the solution shows a change of
colour. The colour change is due to the fact that one tautomer changes over to
the other.
For example, phenolphthalein is tautomeric
mixture of the two forms.
Related Topics