Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations
Secondary Drying - Excipients Used in Parenteral Formulations of biotech Products
Secondary Drying
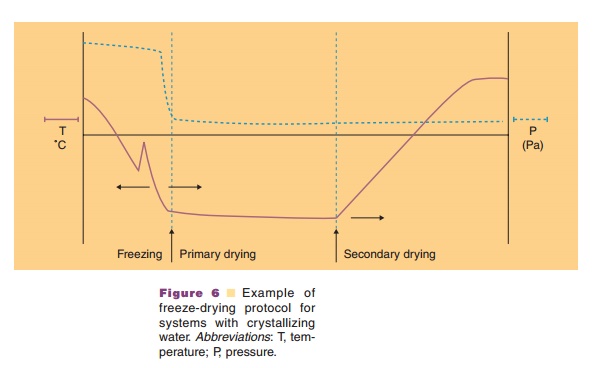
When all frozen or amorphous water that is non-protein and non-excipient bound is removed, the secondary drying step starts (Fig. 6). The end of the primary drying stage is reached when product temperature and shelf temperature become equal, or when the partial water pressure drops (Pikal, 1990a). As long as the “non-bound” water is being removed, the partial water pressure almost equals the total pressure. In the secondary drying stage the tempera-ture is slowly increased to remove “bound” water; the chamber pressure is still reduced. The temperature should stay all the time below the collapse/eutectic temperature, which continues to rise when residual water contents drop. Typically, the secondary drying step ends when the product has been kept at 20 C for some time. The residual water content is a critical, endpoint indicating parameter. Values as low as 1% residual water in the cake have been recommended.
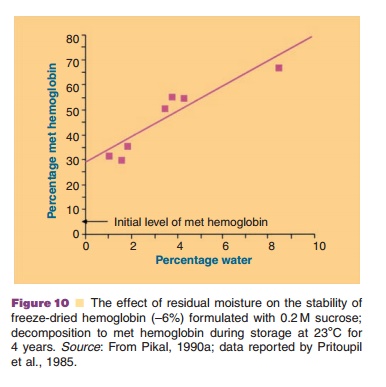
Figure 10 (Pristoupil, 1985; Pikal, 1990a) exemplifies the decreasing stability of freeze-dried hemoglobin with increasing residual water content.
When stored in the presence of reducing lyoprotectants such as glucose and lactose the Maillard reaction may occur: amino groups of the proteins react with the lyoprotectant in the dry state and the cake color turns yellow-brown. The use of non-reducing sugars such as sucrose or trehalose may avoid this problem.
Related Topics