Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Cycles of activity and behavior
Representative life histories of migratory fishes
Representative life histories of migratory fishes
Among vertebrates, fishes stand out in terms of the complexity of their life histories, and migratory fishes have among the most complex life histories. Details of a few of the better known and more interesting species are highlighted below.
Anadromy
Some of the most spectacular examples of highly evolved, complex migrations involve fishes that spawn in fresh water but spend most of their lives at sea. Included among anadromous fishes are lampreys, sturgeons, shads and herrings, salmons and trouts, and striped bass (see Table 23.2). The classic case involves Pacific salmons. Chinook Salmon, Oncorhynchus tshawytscha, can serve as an example. Chinook Salmon spawn in streams of the Pacific northwest coast of North America during the summer and fall, depending on locale (e.g., Quinn et al. 2002). Eggs are buried in gravel nests and hatch into alevins (yolk-sac larvae), which emerge and make their way downstream, eventually transforming into silvery smolts after a few months to 2 years, depending on when they were spawned. Smolts move out into the ocean, grow into juveniles and adults, and move in a series of counterclockwise ellipses through the Northeast Pacific that may carry them as far north and west as the Aleutian Islands of Alaska or as far south as northern California, covering distances of several thousand kilometers (Sockeye Salmon, O. nerka, migrate even farther from land and in larger circles in the open sea, and may cover tens of thousands of kilometers).
After 1–8 years, these adults mature and return to the nearshore area. A coastal migration eventually carries them to the mouth of the river from which they migrated as smolts. They enter this river and work their way up, bypassing hundreds of potentially usable streams and innumerable, seemingly insurmountable barriers such as rapids and waterfalls. In this final stage, they cease feeding, change to a reddish color, and the males develop the characteristic hooknosed appearance known as kipe. They ultimately find the natal stream in which they were hatched – and even the exact place where they were incubated – where they spawn and die (Netboy 1980; Healey & Groot 1987; Brown 1990; Groot & Margolis 1991; Augerot 2005; Quinnet al. 2006).
At each juncture in this complicated journey, fish make directional decisions (e.g., Keefer et al. 2006). Numerous mechanisms, which vary depending on life history stage and habitat, have been proposed to provide directional information for a migrating fry, smolt, juvenile, and adult (Fig. 23.11). Movement by young fish from spawning sites in natal rivers to the ocean involves a combination of responses to light (including a sun compass and discrimination of polarized light), geomagnetic cues, and water currents. The fish must also imprint on (learn) the chemical fingerprint or home stream olfactory bouquet of its home stream and river, or even of multiple, sequential habitats (e.g., Dittman & Quinn 1996; Carruth et al. 2002).
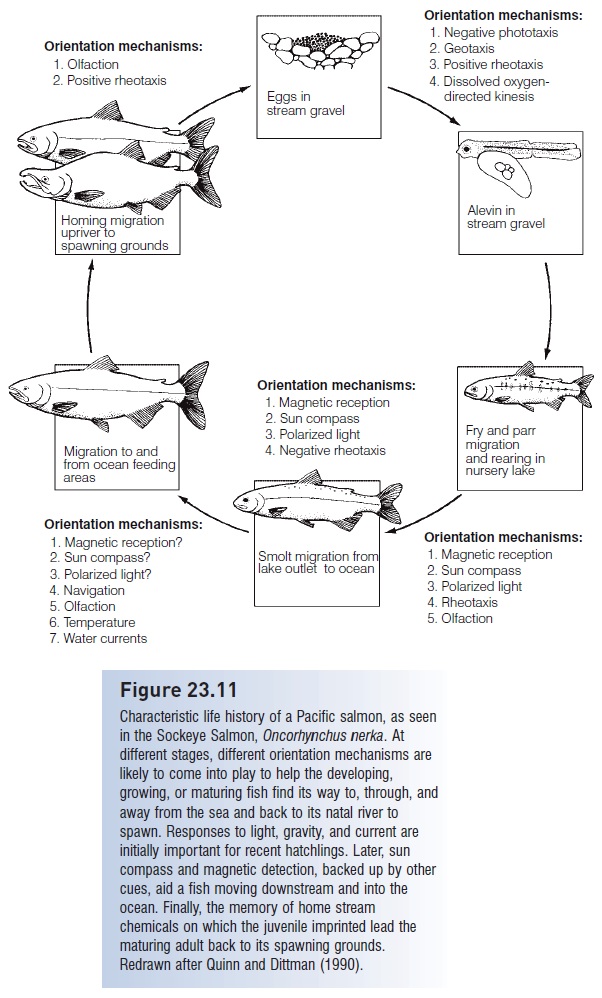
Figure 23.11
Characteristic life history of a Pacific salmon, as seen in the Sockeye Salmon, Oncorhynchus nerka. At different stages, different orientation mechanisms are likely to come into play to help the developing, growing, or maturing fish find its way to, through, and away from the sea and back to its natal river to spawn. Responses to light, gravity, and current are initially important for recent hatchlings. Later, sun compass and magnetic detection, backed up by other cues, aid a fish moving downstream and into the ocean. Finally, the memory of home stream chemicals on which the juvenile imprinted lead the maturing adult back to its spawning grounds. Redrawn after Quinn and Dittman (1990).
Open ocean migration and eventual home stream selection offer very different problems that probably require different orientation systems. Quinn (1982) proposed a combined map–compass–calendar system to explain movements on the high seas. The map would involve learned or genetic knowledge of the distribution of the earth’s magnetic field (which has also been mapped by oceanographers and is predictable). Compass directions, provided by celestial and magnetic cues, can be used to maintain directional
The calendar would require an assessment of day length or change in day length, with input from an endogenous circa-annual clock. Integration of all this information would tell a fish where it was, where it was going, and how long it would take to get there, forming the basis of a navigational system.
Once a maturing adult arrived in the coastline region of its home river, it would shift to an olfactorally guided response to natural chemicals contained in different rivers. Having remembered the chemical fingerprint of its natal stream, it would move upriver and reject any stream mouth it passed that did not have the appropriate bouquet. Upon encountering the correct chemical cues, it would move upcurrent in that system until it arrived at the appropriate spawning site. The olfactory hypothesis has received experimental confi rmation in studies with Coho Salmon, O. kisutch, transplanted into Lake Michigan. Fish were imprinted on synthetic chemicals in hatchery water and released. Eighteen months later, most chemically imprinted salmon that entered streams chose streams containing the synthetic chemicals (Hasler & Scholz 1983; Quinn & Dittman 1990).
Home stream return, perhaps involving olfactory guidance, also occurs in Striped Bass, Morone saxatilis, which forms stocks along the US Atlantic coast that are associated with major river systems. Fish migrate north in the spring and south in the fall along the Atlantic coast, but return each spring to spawn in their natal rivers. American Shad, Alewives, and Blueback Herring also home to their natal rivers to spawn (Boreman & Lewis 1987; Loesch 1987; Quinn & Leggett 1987).
Which is not to say that mistakes do not occur. Although as many as 98.6% of Chinook Salmon may home correctly
to the Cowlitz River in Washington, the same species may show 10–13% straying rates in California rivers. In fact, tagging studies show that as many as 47% of fish may wind up in the wrong, or at least in a non-natal, stream. The pattern of straying is, however, adaptive. High fi delity (low straying) rates characterize species and populations that spawn in large, stable rivers, whereas straying is more common in fish that come primarily from small, unstable rivers with variable flow characteristics, where juvenile survival is also more variable. Straying can then be viewed as an alternative life history trait that functions as a bethedging tactic to insure survival of some offspring in situations where the natal river may become uninhabitable.
Catadromy
Life history variation occurs among salmons and sturgeons, with some species and populations being landlocked or seldom entering the sea. In contrast, all 15 species of the eel family Anguillidae are thought to spawn in the sea but grow up in fresh water. The best known species are the European, Japanese, and American eels, all of which undergo larval and adult migrations of truly epic proportions. The American Eel, Anguilla rostrata, can serve as an example.
American Eels spawn in the Sargasso Sea, an unproductive region of the western Atlantic northeast of Hispaniola and the Bahamas. The exact locale of spawning remained a mystery until the 1920s, when Danish biologist Johannes Schmidt analyzed 25 years of oceanic plankton tows and determined that the smallest eel larvae of both American and European species (known as leptocephali and once thought to be a different species of fish altogether) were captured in this area. Schmidt’s results have been subsequently confi rmed by captures of even smaller larvae (<7 mm long) of both species at the same time from the same locale; American and European Eels spawn in overlapping areas during early spring and then drift northward with major ocean currents. European larvae apparently do not metamorphose until they are 2–3 years old, hence they float past the North American continent. American Eel leptocephali in contrast metamorphose after about 1 year and, using transport mechanisms that remain unresolved, move westward to inshore waters. Interestingly, hybrids between the European and American species stop halfway, in Iceland. Mysteriously, leptocephali are not thought to feed, or at least they have nonfunctional guts during most of their larval phase. Leptocephali are next attracted by the mixture of organic materials dissolved in outflowing fresh waters and migrate upriver, moving by selective tidal stream transport (see above) and transforming into transparent, miniature (50 mm) eels known asglass eels. As they move upriver, they become pigmented and are called elvers.
Elvers grow into juvenile yellow eels that take up residence in fresh water for periods that range from 3 to 40 years, the time depending on sex and latitude. Males are more abundant in southerly latitudes and in estuaries. They never grow larger than 44 cm and usually mature after 3–10 years. Females are likely to be found throughout a river system, from the estuary all the way up to the headwaters. In fact, female American Eels probably have the widest geographic and environmental range of any nonintroduced freshwater fish anywhere in the world. Their habitats include rapidly flowing, clear, headwater streams, large lakes and rivers, underground cave springs, lowland rivers and swamps, down to estuarine saltmarshes. They are found from Iceland to Venezuela, including most Caribbean islands and Bermuda, and range up the Mississippi River to its headwaters and as far west as the Yucatan Peninsula.
Maturation varies as a function of this range. In general, more northerly populations and those farther from the Sargasso Sea contain older, larger (and usually female) animals. Maturation may take from 4 to 13 years at southerly locales and as much as 43 years in Nova Scotia (Jessop 1987). As the animals mature, they turn a silvery-bronze color, the pectoral fins become pointed, the eyes enlarge (particularly in males), and fat stores are accumulated. These nonfeeding, silver eels then migrate back to the Sargasso Sea, migrations beginning earlier for animals traveling farther, which apparently synchronizes the time of arrival at the spawning grounds. Silver eels travel as much as 5000 km to spawn and then apparently die. Conjecture surrounds this stage because no one has seen a fully mature anguillid eel, nor located an adult eel at the presumed spawning grounds (Tesch 1977; McCleave et al. 1987; Helfman et al. 1987; Avise et al. 1990).
Oceanodromy
Oceanodromous fishes migrate within ocean basins, usually in a circuit and usually traveling with major ocean currents. The migration serves to place different life history stages in seasonally appropriate locales. The range may therefore include an area for spawning from which eggs and larvae float to a nursery area, winter and summer feeding areas for juveniles and adults, and also migratory zones through which a stock moves. Juveniles may move between seasonal feeding areas for several years before maturing and migrating to spawning grounds. The great tunas (Thunnus, Scombridae), particularly those living in temperate waters, are representative. Subspecies or stocks have been suggested for different ocean basins in the past, but movement across ocean basins and probable mixing of stocks tends to eliminate genetic differences (e.g., for the highly migratory Albacore Tuna, Thunnus alalunga; Graves & Dizon 1987).
Bluefin tuna are subdivided into Bluefin, Thunnus thynnus, in the Atlantic and Mediterranean; Pacific Bluefin, T. orientalis; and Southern Bluefin, T. maccoyii, off Australia, New Zealand, and South Africa. Bluefin tagged off Florida have been recaptured in Norway, involving a minimum migration distance of 10,000 km. In addition, fish of different sizes have different migratory patterns, and adults of different sizes may spawn at different times and places. In the western North Atlantic, the largest Bluefin (120–900 kg) have a migratory cycle that begins on summer feeding grounds (May to September) over the continental shelf from Cape Hatteras to Nova Scotia. This is followed by fall and winter movements offshore and south to wintering grounds that include the Bahamas, Greater Antilles, and into the Caribbean and Gulf of Mexico. In the spring, the giants move northward in oceanic waters and then onto the continental shelf in late spring and then back to the summer feeding grounds. Spawning occurs in southern waters (Gulf of Mexico, Straits of Florida) in May and June, and in the Mediterranean and Black seas during the warm summer months. Mixing may involve as many as 30% of fish crossing the Atlantic from west to east (mixing from east to west is less well known) (McClane 1974; Richards 1976; Rivas1978; Lutcavage et al. 1999; Block et al. 2001; Block &Stevens 2001).
Differentiation into stocks, some that mix and some that do not, appears common among oceanodromous fishes. Atlantic Herring (Clupea harengus) are subdivided into several spawning groups or stocks (six alone in the Northeast Atlantic) that may be further subdivided into isolated stocks in various estuaries and inlets. Migrations carry different stocks to overlapping feeding areas but spawning occurs at separate times and places. Atlantic Cod (Gadus morhua, Gadidae) and Plaice (Pleuronectes platessa, Pleuronectidae) are also differentiated into several migratory stocks with distinct spawning grounds. Bluefish (Pomatomus saltatrix, Pomatomidae) occur worldwide in warmer oceans except for the eastern Pacific. Schools apparently migrate onshore and offshore with the seasons, perhaps following baitfish. Along the US Atlantic coast, this migration involves an inshore migration in spring and summer and a return to offshore locales in fall and winter, which corresponds with movements of a primary prey species, Menhaden (Brevoortia tyrannus). Bluefish movements occur progressively later as one travels north, and the pattern is complicated by a degree of north–south migration (McKeown 1984; Hersey 1988). Other, larger predators, such as Blue Marlin (Makaira nigricans, Istiophoridae), also are oceanodromous, move seasonally, and form local but wide-ranging stocks.
Related Topics