Chapter: Medical Physiology: Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Release of Energy from the Glucose Molecule by the Glycolytic Pathway
Release of Energy from the Glucose Molecule by the Glycolytic Pathway
Because complete oxidation of 1 gram-molecule of glucose releases 686,000 calories of energy and only 12,000 calories of energy are required to form 1 gram-molecule of ATP, energy would be wasted if glucose were decomposed all at once into water and carbon dioxide while forming only a single ATP mole-cule. Fortunately, all cells of the body contain special protein enzymes that cause the glucose molecule to split a little at a time in many successive steps, so that its energy is released in small packets to form one mole-cule of ATP at a time, forming a total of 38 moles of ATP for each mole of glucose metabolized by the cells.
The next sections describe the basic principles of the processes by which the glucose molecule is pro-gressively dissected and its energy released to form ATP.
Glycolysis and the Formation of Pyruvic Acid
By far the most important means of releasing energy from the glucose molecule is initiated by glycolysis. The end products of glycolysis are then oxidized to provide energy. Glycolysis means splitting of the glucose mole-cule to form two molecules of pyruvic acid.
Glycolysis occurs by 10 successive chemical reactions, shown in Figure 67–5. Each step is catalyzed by at least one specific protein enzyme. Note that glucose is first converted into fructose-1,6-diphosphate and then split into two three-carbon-atom molecules, glyceraldehyde-3-phosphate, each of which is then converted through five additional steps into pyruvic acid.
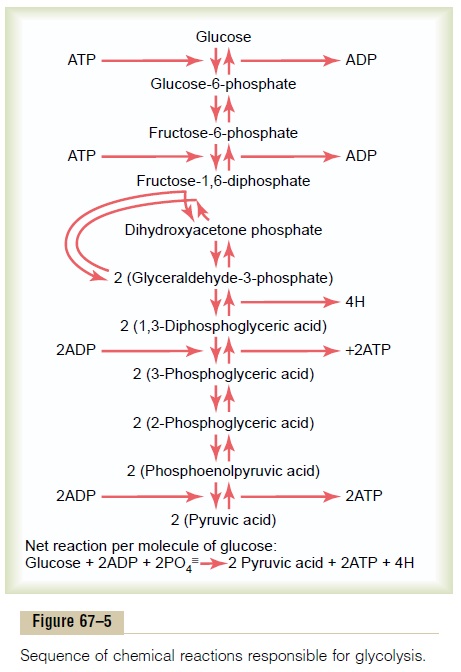
Formation of ATP During Glycolysis. Despite the many chem-ical reactions in the glycolytic series, only a small portion of the free energy in the glucose molecule is released at most steps. However, between the 1,3-diphosphoglyceric acid and the 3-phosphoglyceric acid stages, and again between the phosphoenolpyruvic acid and the pyruvic acid stages, the packets of energy released are greater than 12,000 calories per mole, the amount required to form ATP, and the reactions are coupled in such a way that ATP is formed. Thus, a total of 4 moles of ATP were formed for each mole of fruc-tose-1,6-diphosphate that is split into pyruvic acid.
Yet 2 moles of ATP were required to phosphorylate the original glucose to form fructose-1,6-diphosphate before glycolysis could begin. Therefore, the net gain in ATP molecules by the entire glycolytic process is only 2 moles for each mole of glucose utilized.
This amounts to24,000 calories of energy that becomes transferred to ATP, but during glycolysis, a total of 56,000 calories of energy were lost from the original glucose, giving an overall efficiency for ATP formation of only 43 per cent. The remaining 57 per cent of the energy is lost in the form of heat.
Conversion of Pyruvic Acid to Acetyl Coenzyme A
The next stage in the degradation of glucose is a two-step conversion of the two pyruvic acid molecules from Figure 67–5 into two molecules of acetyl coenzymeA (acetyl-CoA), in accordance with the following reaction:
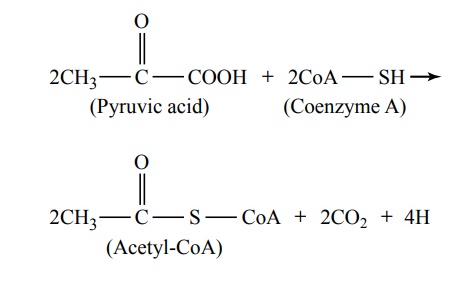
From this reaction, it can be seen that two carbon dioxide molecules and four hydrogen atoms are released, while the remaining portions of the two pyruvic acid molecules combine with coenzyme A, a derivative of the vitamin pantothenic acid, to form two molecules of acetyl-CoA. In this conversion, no ATP is formed, but up to six molecules of ATP are formed when the four released hydrogen atoms are later oxi-dized, as discussed later.
Citric Acid Cycle (Krebs Cycle)
The next stage in the degradation of the glucose mole-cule is called the citric acid cycle (also called the tricar-boxylic acid cycle or Krebs cycle). This is a sequence ofchemical reactions in which the acetyl portion of acetyl-CoA is degraded to carbon dioxide and hydrogen atoms. These reactions all occur in the matrix of themitochondrion. The released hydrogen atoms add tothe number of these atoms that will subsequently be oxidized (as discussed later), releasing tremendous amounts of energy to form ATP.
Figure 67–6 shows the different stages of the chemi-cal reactions in the citric acid cycle. The substances to the left are added during the chemical reactions, and the products of the chemical reactions are shown to the right. Note at the top of the column that the cycle begins with oxaloacetic acid, and at the bottom of the chain of reactions, oxaloacetic acid is formed again. Thus, the cycle can continue over and over.
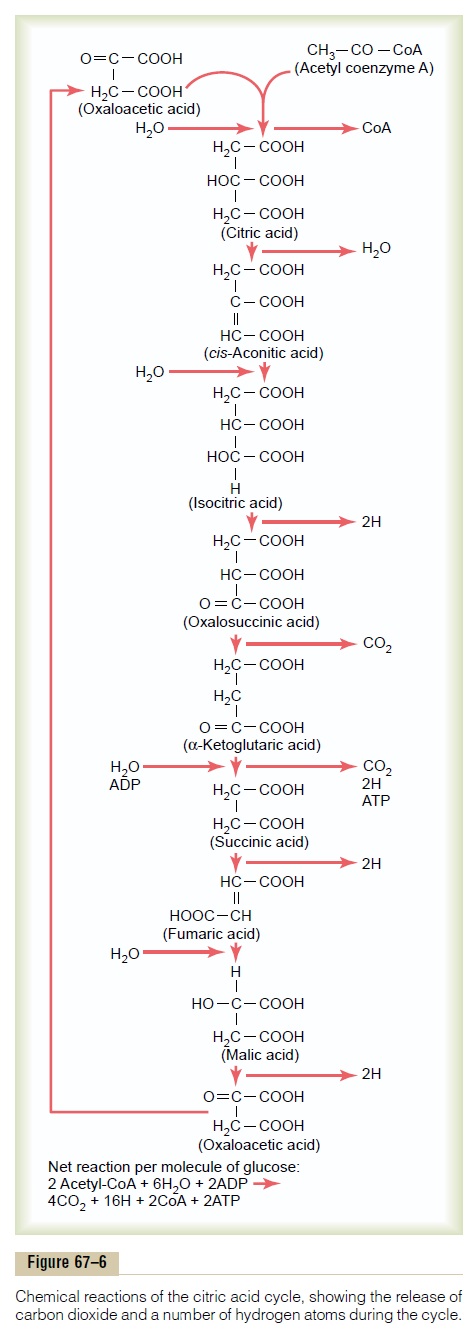
In the initial stage of the citric acid cycle, acetyl-CoA combines with oxaloacetic acid to form citric acid. The coenzyme A portion of the acetyl-CoA is released and can be used again and again for the formation of still more quantities of acetyl-CoA from pyruvic acid.
The acetyl portion, however, becomes an integral part of the citric acid molecule. During the successive stages of the citric acid cycle, several molecules of water are added, as shown on the left in the figure, and carbon dioxide and hydrogen atoms are released at other stages in the cycle, as shown on the right in the figure.
The net results of the entire citric acid cycle are given in the explanation at the bottom of Figure 67–6, demon-strating that for each molecule of glucose originally metabolized, 2 acetyl-CoA molecules enter into the citric acid cycle, along with 6 molecules of water. These are then degraded into 4 carbon dioxide molecules, 16 hydrogen atoms, and 2 molecules of coenzyme A. Two molecules of ATP are formed, as follows.
Formation of ATP in the Citric Acid Cycle. Not a great amountof energy is released during the citric acid cycle itself; in only one of the chemical reactions—during the change from a-ketoglutaric acid to succinic acid—is a molecule of ATP formed. Thus, for each molecule of glucose metabolized, two acetyl-CoA molecules pass through the citric acid cycle, each forming a molecule of ATP, or a total of two molecules of ATP formed.
Function of Dehydrogenases and Nicotinamide Adenine Dinu-cleotide in Causing Release of Hydrogen Atoms in the Citric Acid Cycle. As already noted at several points in this discus-sion, hydrogen atoms are released during different chemical reactions of the citric acid cycle—4 hydrogen atoms during glycolysis, 4 during formation of acetyl-CoA from pyruvic acid, and 16 in the citric acid cycle; this makes a total of 24 hydrogen atoms released for each original molecule of glucose. However, the hydrogenatoms are not simply turned loose in the intracellular fluid. Instead, they are released in packets of two, and in each instance, the release is catalyzed by a specific protein enzyme called a dehydrogenase. Twenty of the 24 hydrogen atoms immediately combine with nicoti-namide adenine dinucleotide (NAD+), a derivative of the vitamin niacin, in accordance with the following reaction:
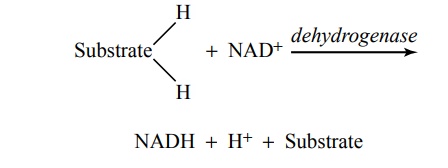
This reaction will not occur without intermediation of the specific dehydrogenase or without the availability of NAD+ to act as a hydrogen carrier. Both the free hydro-gen ion and the hydrogen bound with NAD+ subse-quently enter into multiple oxidative chemical reactions that form tremendous quantities of ATP, as discussed later.
The remaining four hydrogen atoms released during the breakdown of glucose—the four released during the citric acid cycle between the succinic and fumaric acid stages—combine with a specific dehydrogenase but are not subsequently released to NAD+. Instead, they pass directly from the dehydrogenase into the oxidative process.
Function of Decarboxylases in Causing Release of Carbon Dioxide.
Referring again to the chemical reactions of the citric acid cycle, as well as to those for the formation of acetyl-CoA from pyruvic acid, we find that there are three stages in which carbon dioxide is released. To cause the release of carbon dioxide, other specific protein enzymes, called decarboxylases, split the carbon dioxide away from the substrate. The carbon dioxide is then dissolved in the body fluids and transported to the lungs, where it is expired from the body.
Formation of Large Quantities of ATP by Oxidation of Hydrogen (the Process of Oxidative Phosphorylation)
Despite all the complexities of (1) glycolysis, (2) the citric acid cycle, (3) dehydrogenation, and (4) decar-boxylation, pitifully small amounts of ATP are formed during all these processes—only two ATP molecules in the glycolysis scheme and another two in the citric acid cycle for each molecule of glucose metabolized. Instead, almost 90 per cent of the total ATP created through glucose metabolism is formed during subsequent oxi-dation of the hydrogen atoms that were released at early stages of glucose degradation. Indeed, the princi-pal function of all these earlier stages is to make the hydrogen of the glucose molecule available in forms that can be oxidized.
Oxidation of hydrogen is accomplished, as illustrated in Figure 67–7, by a series of enzymatically catalyzed reactions in the mitochondria. These reactions (1) split each hydrogen atom into a hydrogen ion and an elec-tron and (2) use the electrons eventually to combine dis-solved oxygen of the fluids with water molecules to form hydroxyl ions. Then the hydrogen and hydroxyl ions combine with each other to form water. During
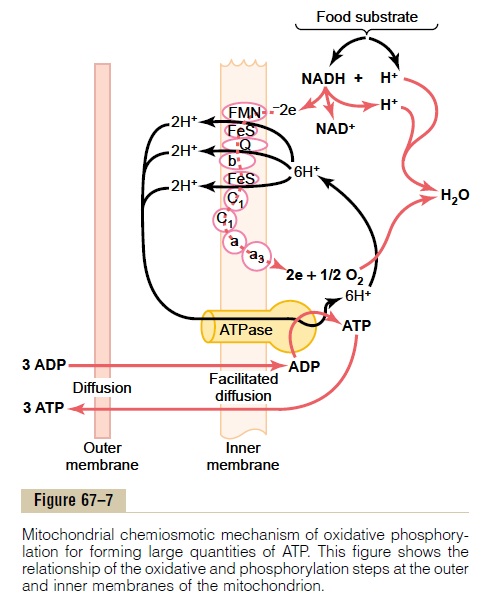
this sequence of oxidative reactions, tremendous quantities of energy are released to form ATP. Forma-tion of ATP in this manner is called oxidative phospho-rylation. This occurs entirely in the mitochondria by ahighly specialized process called the chemiosmoticmechanism.
Chemiosmotic Mechanism of the Mitochondria to Form ATP
Ionization of Hydrogen, the Electron Transport Chain, and Forma- tion of Water. Thefirst step in oxidative phosphorylationin the mitochondria is to ionize the hydrogen atoms that have been removed from the food substrates. As described earlier, these hydrogen atoms are removed in pairs: one immediately becomes a hydrogen ion, H+; the other combines with NAD+ to form NADH. The upper portion of Figure 67–7 shows the subsequent fate of the NADH and H+. The initial effect is to release the other hydrogen atom from the NADH to form another hydro-gen ion, H+; this process also reconstitutes NAD+ that will be reused again and again.
The electrons that are removed from the hydrogen atoms to cause the hydrogen ionization immediately enter an electron transport chain of electron acceptors that are an integral part of the inner membrane (the shelf membrane) of the mitochondrion. The electron acceptors can be reversibly reduced or oxidized by accepting or giving up electrons. The important members of this electron transport chain include flavo-protein, several iron sulfide proteins, ubiquinone, and cytochromes B, C1, C, A, and A3. Each electron is shut-tled from one of these acceptors to the next until it finally reaches cytochrome A3, which is called cytochrome oxidase because it is capable of giving uptwo electrons and thus reducing elemental oxygen to form ionic oxygen, which then combines with hydrogen ions to form water.
Thus, Figure 67–7 shows the transport of electrons through the electron chain and then their ultimate use by cytochrome oxidase to cause the formation of water molecules. During the transport of these elec-trons through the electron transport chain, energy is released that is used to cause the synthesis of ATP, as follows.
Pumping of Hydrogen Ions into the Outer Chamber of the Mito- chondrion, Caused by the Electron Transport Chain. As theelectrons pass through the electron transport chain, large amounts of energy are released. This energy is used to pump hydrogen ions from the inner matrix of the mitochondrion (to the right in Figure 67–7) into the outer chamber between the inner and outer mitochon-drial membranes (to the left). This creates a high con-centration of positively charged hydrogen ions in this chamber; it also creates a strong negative electrical potential in the inner matrix.
Formation of ATP. The next step in oxidative phosphory-lation is to convert ADP into ATP. This occurs in con-junction with a large protein molecule that protrudes all the way through the inner mitochondrial membrane and projects with a knoblike head into the inner mito-chondrial matrix. This molecule is an ATPase, the phys-ical nature of which is shown in Figure 67–7. It is called ATP synthetase.
The high concentration of positively charged hydro-gen ions in the outer chamber and the large electrical potential difference across the inner membrane cause the hydrogen ions to flow into the inner mitochondrial matrix through the substance of the ATPase molecule. In doing so, energy derived from this hydrogen ion flow is used by ATPase to convert ADP into ATP by combin-ing ADP with a free ionic phosphate radical (Pi), thus adding another high-energy phosphate bond to the molecule.
The final step in the process is transfer of ATP from the inside of the mitochondrion back to the cell cyto-plasm. This occurs by facilitated diffusion outward through the inner membrane and then by simple dif-fusion through the permeable outer mitochondrial membrane. In turn, ADP is continually transferred in the other direction for continual conversion into ATP. For each two electrons that pass through the entire elec-tron transport chain (representing the ionization of two hydrogen atoms), up to three ATP molecules are synthesized.
Related Topics