Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Relation between Kp and Kc
Relation between Kp and Kc
Let us consider the general reaction in which all reactants and products are ideal gases.
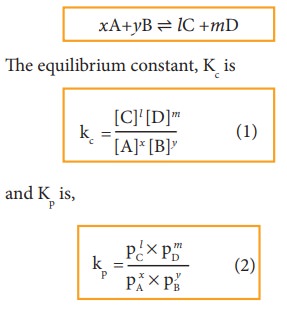
The ideal gas equation is
Since
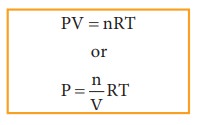
Active mass = molar concentration = n/V
P = active mass × RT
Based on the above expression the partial pressure of the reactants and products can be expressed as,
pAx = [A]x [RT]x
pBy = [B]y [RT]y
pC1 = [C]l [RT]l
pmD = [D]m [RT]m
On substitution in Eqn. 2,

By comparing equation (1) and (4), we get
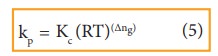
where,
Δng is the difference between the sum of number of moles of products and the sum of number of moles of reactants in the gas phase.
The following relations become immediately obvious.
When Δng = 0
Kp = Kc (RT)0 = Kc
Example:
H2(g) + I2(g) ⇌ 2HI (g)
N2 (g) +O2 (g) ⇌ 2NO(g)
When Δng = +ve
Kp = Kc (RT)+ve
Kp>Kc
2NH3(g) ⇌ N2 (g) + 3H2 (g)
PCI5 (g) ⇌ PCl3 (g) + Cl2 (g)
When Δng = -ve
Kp = Kc (RT)-ve
Kp< Kc
Example:
2H2(g) + O2(g) ⇌ 2H2O (g)
2SO2(g) + O2(g) ⇌ 2SO3 (g)
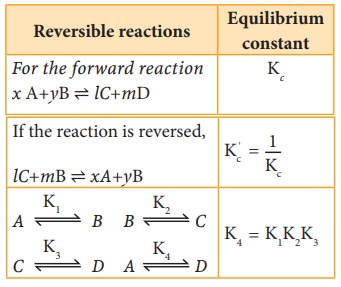
Table 8.1 Relation between equilibrium constants for some reversible reactions
Related Topics