Chapter: Medical Physiology: Adrenocortical Hormones
Regulation of Cortisol Secretion by Adrenocorticotropic Hormone from the Pituitary Gland
Regulation of Cortisol Secretion by Adrenocorticotropic Hormone from the Pituitary Gland
ACTH Stimulates Cortisol Secretion. Unlike aldosteronesecretion by the zona glomerulosa, which is controlled mainly by potassium and angiotensin acting directly on the adrenocortical cells, almost no stimuli have direct control effects on the adrenal cells that secrete cortisol. Instead, secretion of cortisol is controlled almost entirely by ACTH secreted by the anterior pituitary gland. This hormone, also called corticotropin or adrenocorticotropin, also enhances the production of adrenal androgens.
Chemistry of ACTH. ACTH has been isolated in pureform from the anterior pituitary. It is a large polypep-tide, having a chain length of 39 amino acids. A smaller polypeptide, a digested product of ACTH having a chain length of 24 amino acids, has all the effects of the total molecule.
ACTH Secretion Is Controlled by Corticotropin-Releasing Factor from the Hypothalamus. In the same way that other pitu-itary hormones are controlled by releasing factors from the hypothalamus, an important releasing factor also controls ACTH secretion. This is called corti-cotropin-releasing factor(CRF). It is secreted into theprimary capillary plexus of the hypophysial portal system in the median eminence of the hypothalamus and then carried to the anterior pituitary gland, where it induces ACTH secretion. CRF is a peptide com-posed of 41 amino acids. The cell bodies of the neurons that secrete CRF are located mainly in the paraven-tricular nucleus of the hypothalamus. This nucleus in turn receives many nervous connections from the limbic system and lower brain stem.
The anterior pituitary gland can secrete only minute quantities of ACTH in the absence of CRF. Instead, most conditions that cause high ACTH secretory rates initiate this secretion by signals that begin in the basal regions of the brain, including the hypothalamus, and are then transmitted by CRF to the anterior pituitary gland.
ACTH Activates Adrenocortical Cells to Produce Steroids by Increasing Cyclic Adenosine Monophosphate (cAMP). Theprincipal effect of ACTH on the adrenocortical cells is to activate adenylyl cyclase in the cell membrane. This then induces the formation of cAMP in the cell cyto-plasm, reaching its maximal effect in about 3 minutes.
The cAMP in turn activates the intracellular enzymes that cause formation of the adrenocortical hormones.
This is another example of cAMP as a second messen-ger signal system.
The most important of all the ACTH-stimulated steps for controlling adrenocortical secretion is acti-vation of the enzyme protein kinase A, whichcausesinitial conversion of cholesterol to pregnenolone. Thisinitial conversion is the “rate-limiting” step for all the adrenocortical hormones, which explains why ACTH normally is necessary for any adrenocortical hormones to be formed. Long-term stimulation of the adrenal cortex by ACTH not only increases secretory activity but also causes hypertrophy and proliferation of the adrenocortical cells, especially in the zona fasciculata and zona reticularis, where cortisol and the androgens are secreted.
Physiologic Stress Increases ACTH and Adrenocortical Secretion
As pointed out earlier, almost any type of physical or mental stress can lead within minutes to greatly enhanced secretion of ACTH and conse-quently cortisol as well, often increasing cortisol secre-tion as much as 20-fold. This effect was demonstrated by the rapid and strong adrenocortical secretory responses after trauma shown in Figure 77–5.
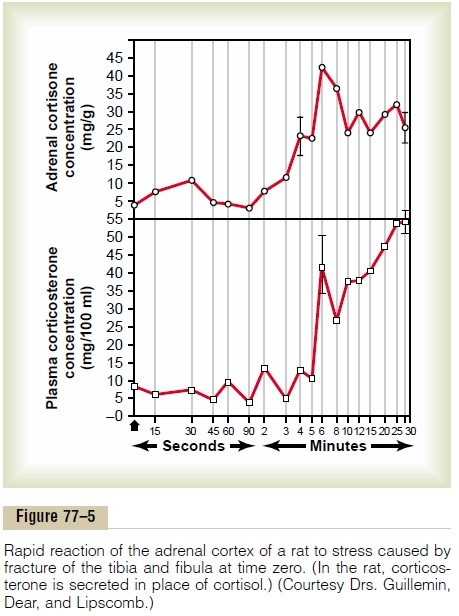
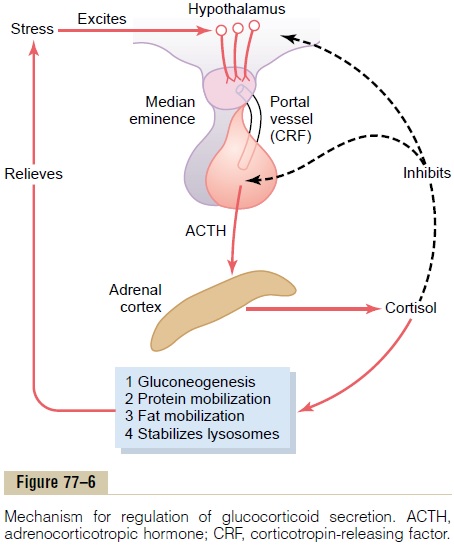
Pain stimuli caused by physical stress or tissue damage are transmitted first upward through the brain stem and eventually to the median eminence of the hypothalamus, as shown in Figure 77–6. Here CRF is secreted into the hypophysial portal system. Within minutes the entire control sequence leads to large quantities of cortisol in the blood.
Mental stress can cause an equally rapid increase in ACTH secretion. This is believed to result from increased activity in the limbic system, especially in the region of the amygdala and hippocampus, both of which then transmit signals to the posterior medial hypothalamus.
Inhibitory Effect of Cortisol on the Hypothalamus and on the Anterior Pituitary to Decrease ACTH Secretion. Cortisol hasdirect negative feedback effects on (1) the hypothala-mus to decrease the formation of CRF and (2) the anterior pituitary gland to decrease the formation of ACTH. Both of these feedbacks help regulate the plasma concentration of cortisol. That is, whenever the cortisol concentration becomes too great, the feedbacks automatically reduce the ACTH toward a normal control level.
Summary of the Cortisol Control System
Figure 77–6 shows the overall system for control of cortisol secretion. The key to this control is the exci-tation of the hypothalamus by different types of stress.
Stress stimuli activate the entire system to cause rapid release of cortisol, and the cortisol in turn initiates a series of metabolic effects directed toward relieving the damaging nature of the stressful state.
There is also direct feedback of the cortisol to both the hypothalamus and the anterior pituitary gland to decrease the concentration of cortisol in the plasma at times when the body is not experiencing stress.
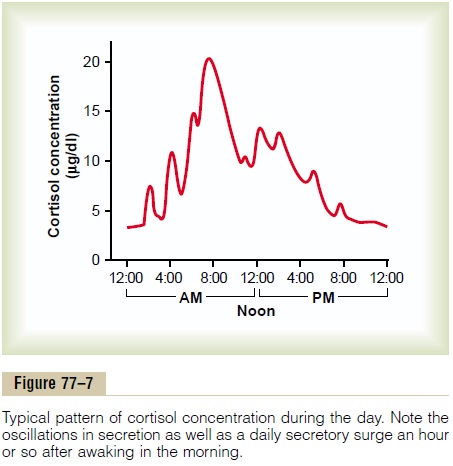
However, the stress stimuli are the prepotent ones; they can always break through this direct inhibitory feedback of cortisol, causing either periodic exacerba-tions of cortisol secretion at multiple times during the day (Figure 77–7) or prolonged cortisol secretion in times of chronic stress.
Circadian Rhythm of Glucocorticoid Secretion. The secretoryrates of CRF, ACTH, and cortisol are high in the early morning but low in the late evening, as shown in Figure 77–7; the plasma cortisol level ranges between a high of about 20 mg/dl an hour before arising in the morning and a low of about 5 mg/dl around midnight. This effect results from a 24-hour cyclical alteration in the signals from the hypothalamus that cause cortisol secretion. When a person changes daily sleeping habits, the cycle changes correspondingly. Therefore, measurements of blood cortisol levels are meaningful only when expressed in terms of the time in the cycle at which the measurements are made.
Synthesis and Secretion of ACTH in Association with Melanocyte-Stimulating Hormone, Lipotropin, and Endorphin
When ACTH is secreted by the anterior pituitary gland, several other hormones that have similar chem-ical structures are secreted simultaneously. The reason for this is that the gene that is transcribed to form the RNA molecule that causes ACTH synthesis initially causes the formation of a considerably larger protein, a preprohormone called proopiomelanocortin(POMC), which is the precursor of ACTH as wellas several other peptides, including melanocyte-stimulating hormone (MSH), b-lipotropin, b-endor-phin, and a few others (Figure 77–8). Under normalconditions, none of these hormones is secreted in enough quantity by the pituitary to have a significant effect on the human body, but when the rate of secre-tion of ACTH is high, as may occur in Addison’s disease, formation of some of the other POMC-derived hormones may also be increased.
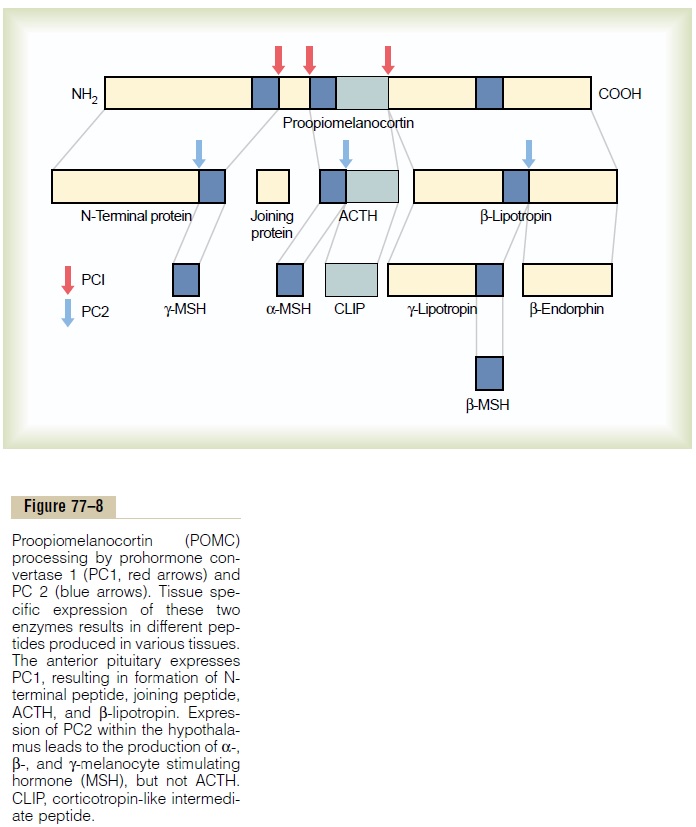
The POMC gene is actively transcribed in several tissues, including the corticotroph cells of the anterior pituitary, POMC neurons in the arcuate nucleus of the hypothalamus, cells of the dermis, and lymphoid tissue. In all of these cell types, POMC is processed to form a series of smaller peptides. The precise type of POMC-derived products from a particular tissue depends on the type of processing enzymes present in the tissue. Thus, pituitary corticotroph cells express prohormone convertase 1 (PC1), but not PC2, result-ing in the production of N-terminal peptide, joining peptide, ACTH, b-endorphin, and b-lipotropin. In the hypothalamus, the expression of PC2 leads to the production of a-, b-, and g-MSH, but not ACTH. A-MSH formed by neurons of the hypothalamus plays a major role in appetite regulation.
In melanocytes located in abundance between the dermis and epidermis of the skin, MSH stimulates formation of the black pigment melanin and disperses it to the epidermis. Injection of MSH into a person over 8 to 10 days can greatly increase darkening of the skin. The effect is much greater in people who have genetically dark skins than in light-skinned people.
In some lower animals, an intermediate “lobe” of the pituitary gland, called the pars intermedia, is highly developed, lying between the anterior and posterior pituitary lobes. This lobe secretes an especially large amount of MSH. Furthermore, this secretion is independently controlled by the hypothalamus in response to the amount of light to which the animal is exposed or in response to other environmental factors. For instance, some arctic animals develop darkened fur in the summer and yet have entirely white fur in the winter.
ACTH, because it contains an MSH sequence, has about 1/30 as much melanocyte-stimulating effect as MSH. Furthermore, because the quantities of pure MSH secreted in the human being are extremely small, whereas those of ACTH are large, it is likely that ACTH normally is more important than MSH in determining the amount of melanin in the skin.
Related Topics