Chapter: Clinical Anesthesiology: Anesthetic Management: Neurophysiology & Anesthesia
Regulation of Cerebral Blood Flow
REGULATION OF CEREBRAL BLOOD FLOW
1. Cerebral Perfusion Pressure
Cerebral perfusion pressure (CPP) is the
dif-ference between mean arterial pressure (MAP)cand
intracranial pressure (ICP) (or central venous pressure [CVP], if it is greater
than ICP). MAP – ICP (or CVP) = CPP. CPP is normally
80–100 mm Hg. Moreover, because ICP is normally less than 10 mm Hg, CPP is
primarily dependent on MAP.
Moderate to severe increases in ICP (>30 mm Hg) can compromise CPP and CBF, even in the presence of a
normal MAP. Patients with CPP values less than 50 mm Hg often show slowing on
the EEG, whereas those with a CPP between 25 and 40 mm Hg typically have a flat
EEG. Sustained perfusion pressures less than 25 mm Hg may result in
irrevers-ible brain damage.
2. Autoregulation
Much like the heart and kidneys, the brain nor-mally tolerates a
wide range of blood pressure, with little change in blood flow. The cerebral
vasculature rapidly (10–60 s) adapts to changes in CPP. Decreases in CPP result
in cerebral vasodila-tion, whereas elevations induce vasoconstriction. In
normal individuals, CBF remains nearly con-stant between MAPs of about 60 and
160 mm Hg (Figure26–2). Beyond these
limits, blood flow becomes pressure dependent. Pressures above 150–160 mm Hg
can disrupt the blood–brain bar-rier and
may result in cerebral edema and hemorrhage. The
cerebral autoregulation curve (Figure 26–2) is shifted to the right in patients
with chronic
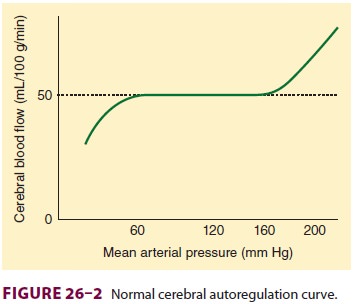
arterial hypertension. Both upper and lower limits are shifted.
Flow becomes more pressure dependent at low “normal” arterial pressures in
return for cerebral protection at higher arterial pressures. Studies suggest
that long-term antihypertensive therapy can restore cerebral autoregulation
limits toward normal.
Both myogenic and metabolic mechanisms may explain cerebral
autoregulation. Myogenic mechanisms involve an intrinsic response of smooth
muscle cells in cerebral arterioles to changes in MAP. Metabolic mechanisms
indicate that cere-bral metabolic demands determine arteriolar tone. Thus, when
tissue demand exceeds blood flow, the release of tissue metabolites causes
vasodilation and increases flow. Whereas hydrogen ions were once thought to
mediate this response, other metabolites are likely involved.
3. Extrinsic Mechanisms
Respiratory Gas Tensions
The most important extrinsic influences
on CBF are respiratory gas tensions particularly Paco2. CBF is directly proportionate to Paco2 between tensions of 20 and 80 mm Hg (Figure26–3).
Blood flow changes approximately 1–2 mL/100 g/min per mm Hg change in Paco2. This effect is almost immediate and is thought to
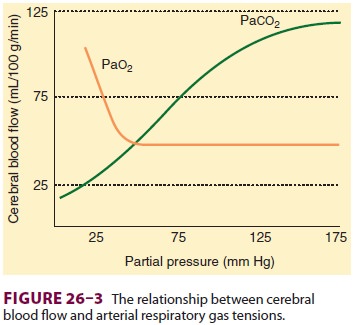
be secondary to changes in the pH of CSF and cerebral tissue.
Because ions do not readily cross the blood–brain barrier but CO 2 does, acute changes in Paco2 but not HCO3– affect CBF. Thus,
acute metabolic acidosis has little effect on CBF because hydrogen ions (H+) cannot readily cross the blood–brain barrier. After 24–48 hr,
CSF HCO3– concentration adjusts to compensate for the change in Paco2, so that the effects
of hypocapnia and hypercapnia are diminished. Marked hyper-ventilation (Paco2< 20 mm Hg) shifts the oxygen–
hemoglobin dissociation curve to the left, and, with changes in CBF, may result
in EEG changes suggestive of cerebral impairment, even in normal individuals.
Only marked changes in Pao2 alter CBF. Whereas hyperoxia may be associated with only minimal
decreases (–10%) in CBF, severe hypox-emia (Pao2< 50 mm Hg) greatly increases CBF (Figure 26–3).
Temperature
CBF changes 5% to 7% per 1°C change in
tem-perature. Hypothermia decreases both CMRand CBF, whereas hyperthermia has
the reverse effect. Between 17°C and 37°C, the Q10 for humans is approximately
2—that is, for every 10° increase in temperature, the CMR doubles. Conversely,
the CMR decreases by 50% if the temperature of the brain falls by 10°C (eg,
from 37°C to 27°C) and another 50% if the temperature decreases from 27°C to
17°C. At 20°C, the EEG is isoelectric, but further decreases in temperature
continue to reduce CMR throughout the brain. Hyperthermia (above 42°C) may
result in neuronal cell injury.
Viscosity
The most important determinant of blood
viscosity is hematocrit. A decrease in hematocrit decreases viscosity and can
improve CBF; unfortunately, a reduction in hematocrit also decreases the
oxygen-carrying capacity and thus can potentially impair oxygen delivery.
Elevated hematocrit, as may be seen with marked polycythemia, increases blood
viscosity and can reduce CBF. Some studies suggest that optimal cerebral oxygen
delivery may occur at hematocrits of approximately 30%.
Autonomic Influences
Intracranial vessels are innervated by
the sympa-thetic (vasoconstrictive) and parasympathetic (vaso-dilatory)
systems, Intense sympathetic stimulation induces vasoconstriction in these
vessels, which can limit CBF. Autonomic innervation may also play an important
role in cerebral vasospasm following brain injury and stroke.
Related Topics