Chapter: Medical Surgical Nursing: Fluid and Electrolytes: Balance and Distribution
Regulation of Body Fluid Compartments
REGULATION
OF BODY FLUID COMPARTMENTS
Osmosis and Osmolality
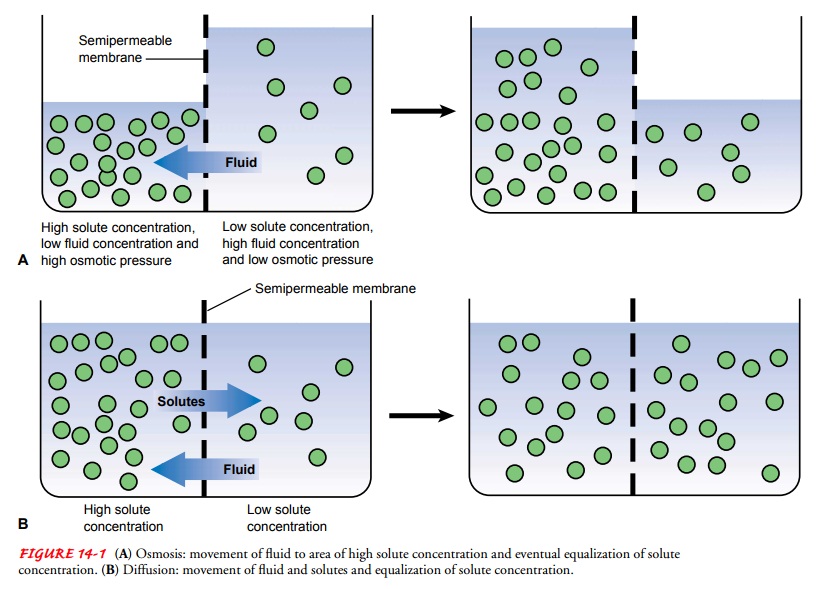
When two different
solutions are separated by a membrane that is impermeable to the dissolved
substances, fluid shifts through the membrane from the region of low solute
concentration to the region of high solute concentration until the solutions
are of equal concentration; this diffusion of water caused by a fluid
con-centration gradient is known as osmosis
(Fig. 14-1A). The mag-nitude of this
force depends on the number of particles dissolved in the solutions, not on
their weights. The number of dissolved particles contained in a unit of fluid
determines the osmolality of a solution, which influences the movement of fluid
between the fluid compartments. Tonicity
is the ability of all the solutes to cause an osmotic driving force that
promotes water movement from one compartment to another (Porth, 2002). The
control of tonicity determines the normal state of cellular hydration and cell
size. Sodium, mannitol, glucose, and sorbitol are effective os-moles (capable
of affecting water movement). Three other terms are associated with osmosis:
osmotic pressure, oncotic pressure, and osmotic diuresis.
•
Osmotic pressure is the amount of hydrostatic
pressure needed to stop the flow of water by osmosis. It is primarily
determined by the concentration of solutes.
•
Oncotic pressure is the osmotic pressure exerted by
proteins (eg, albumin).
•
Osmotic diuresis occurs when the urine output
increases due to the excretion of substances such as glucose, mannitol, or
contrast agents in the urine.
Diffusion
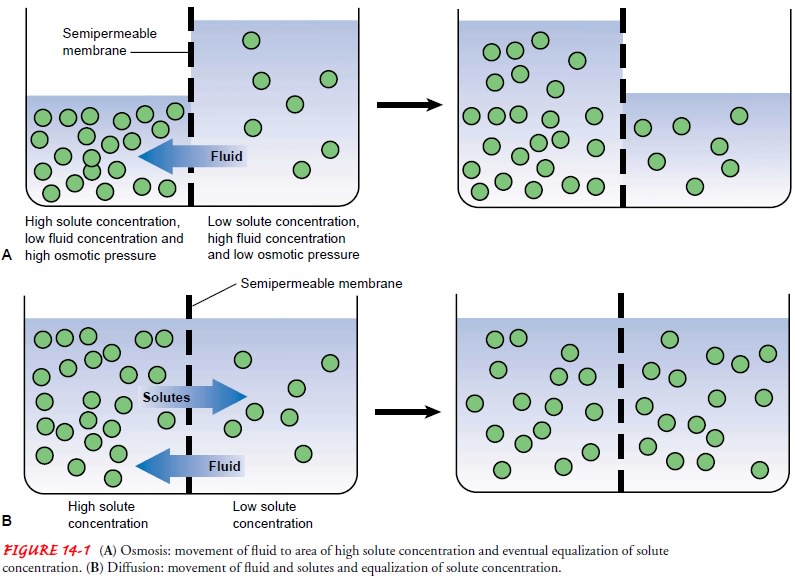
Diffusion is the natural tendency of a substance to
move from anarea of higher concentration to one of lower concentration (see
Fig. 14-1B). It occurs through the
random movement of ions and molecules. Examples of diffusion are the exchange
of oxygen and carbon dioxide between the pulmonary capillaries and alveoli and
the tendency of sodium to move from the ECF compartment, where the sodium
concentration is high, to the ICF, where its concentration is low.
Filtration
Hydrostatic pressure in the capillaries tends to filter fluid out of the
vascular compartment into the interstitial fluid. Movement of water and solutes
occurs from an area of high hydrostatic pres-sure to an area of low hydrostatic
pressure. Filtration allows the kidneys to filter 180 L of plasma per day.
Another example of fil-tration is the passage of water and electrolytes from
the arterial capillary bed to the interstitial fluid; in this instance, the
hydro-static pressure is furnished by the pumping action of the heart.
Sodium–Potassium Pump
As stated earlier, the sodium concentration is greater in the ECF than in the ICF, and because of this, sodium tends to enter the cell by diffusion. This tendency is offset by the sodium–potassium pump, which is located in the cell membrane and actively moves sodium from the cell into the ECF. Conversely, the high intra-cellular potassium concentration is maintained by pumping potas-sium into the cell. By definition, active transport implies that energy must be expended for the movement to occur against a concentration gradient.
Related Topics