Chapter: Organic Chemistry: Alkenes and alkynes
Reduction of alkynes
REDUCTION OF ALKYNES
Key Notes
Hydrogenation
Alkynes
are reduced to alkanes with hydrogen gas over a platinum catalyst. Two
molecules of hydrogen are added and the reaction goes through an alkene
intermediate. The reaction can be stopped at the alkene stage if a less active
or ‘poisoned’ catalyst is used. The stereochemistry of the alkene from such a
reaction is Z since both hydrogen
atoms are added to the same side of the alkyne.
Dissolving metal reduction
Alkynes
can also be reduced to E-alkenes
using sodium or lithium in liquid ammonia.
Hydrogenation
Alkynes react with hydrogen gas in the presence
of a metal catalyst in a process known as hydrogenation – an example of a reduction reaction. With a fully active
catalyst such as platinum metal, two molecules of hydrogen are added to produce
an alkane (Fig. 1).
The reaction involves the addition of one
molecule of hydrogen to form an alkene intermediate which then reacts with a
second molecule of hydrogen to form the alkane. With less active catalysts, it
is possible to stop the reaction at the alkene stage. In particular, Z-alkenes can be synthesized from
alkynes by reaction with hydrogen gas and Lindlar’s catalyst (Fig. 2). This catalyst consists of
metallic palladium deposited on calcium carbonate which is then treated with
lead acetate and quinoline. The latter treatment ‘poisons’ the catalyst such
that the alkyne reacts with hydrogen to give an alkene, but does not react
further. Since the start-ing materials are absorbed onto the catalytic surface,
both hydrogens are added to the same side of the molecule to produce the Z isomer.
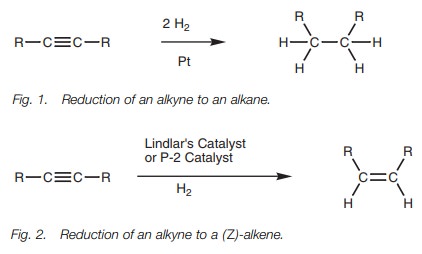
An alternative catalyst which achieves the same
result is nickel boride (Ni2B) – the P-2 catalyst.
Dissolving metal reduction
Reduction of an alkyne to an E-alkene can be achieved if the alkyne
is treated with lithium or sodium metal in ammonia at low temperatures (Fig. 3). This is known as a dissolving
metal reduction.
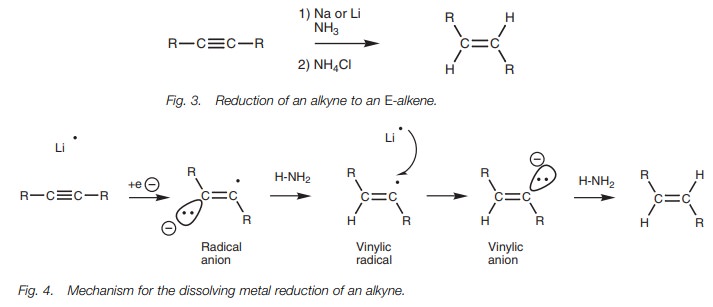
In this reaction, the alkali metal donates its
valence electron to the alkyne to produce a radical anion (Fig. 4). This in turn removes a proton from ammonia to produce a
vinylic radical which receives an electron from a second alkali metal to
produce a trans-vinylic anion. This
anion then removes a proton from a second molecule of ammonia and produces the trans- or E-alkene. Note that half curly arrows are used in the mechanism
since this is a radical reaction involving the movement of single electrons.
Related Topics