Chemical Kinetics - Rate of a chemical reaction | 12th Chemistry : UNIT 7 : Chemical Kinetics
Chapter: 12th Chemistry : UNIT 7 : Chemical Kinetics
Rate of a chemical reaction
Rate
of a chemical reaction:
A rate is a change in a
particular variable per unit time. You have already learnt in physics that
change in the displacement of a particle per unit time gives its velocity.
Similarly in a chemical reaction, the change in the concentration of the
species involved in a chemical reaction per unit time gives the rate of a
reaction.
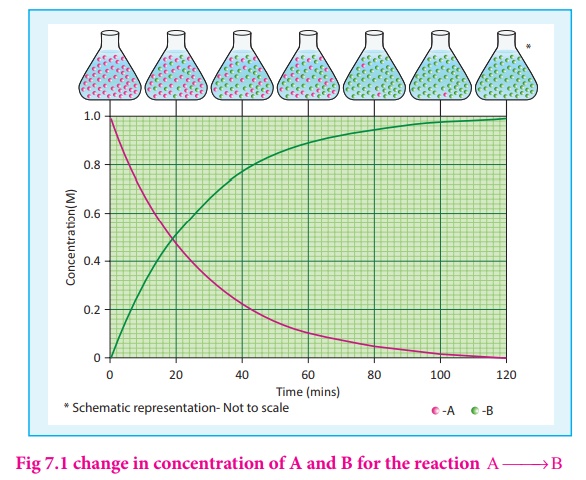
Let us consider a simple
general reaction
A→ B
The concentration of the
reactant ([A]) can be measured at different time intervals. Let the
concentration of A at two different times t2 and t2 , (t2>t1)
be [A1] and [A2] respectively. The rate of the reaction
can be expressed as
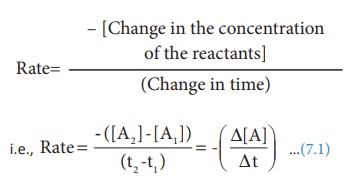
During the reaction, the
concentration of the reactant decreases i.e. [A2 ] < [A1]
and hence the change in concentration [A2 ] - [A1] gives
a negative value. By convention the reaction rate is a positive one and hence a
negative sign is introduced in the rate expression (equation 7.1)
If the reaction is
followed by measuring the product concentration, the rate is given by (Δ[B] / Δ
t) since [B ]>[B ] 2 1 , no minus sign is required here.
Unit of rate of a reaction:
unit of rate = unit of
concentration / unit of time

Usually, concentration
is expressed in number of moles per litre and time is expressed in seconds and
therefore the unit of the rate of a reaction is mol L-1s-1 . Depending upon the
nature of the reaction, minute, hour, year etc can also be used.
For a gas phase
reaction, the concentration of the gaseous species is usually expressed in
terms of their partial pressures and in such cases the unit of reaction rate is
atm s-1 .
1. Stoichiometry and rate of a reaction:
In a reaction A → B ,
the stoichiometry of both reactant and product are same, and hence the rate of
disappearance of reactant (A) and the rate of appearance of product (B) are
same.
Now, let us consider a
different reaction
A→2B
In this case, for every
mole of A, that disappears two moles of B appear, i.e., the rate of formation
of B is twice as fast as the rate of disappearance of A. therefore, the rate of
the reaction can be expressed as below

For a general reaction,
the rate of the reaction is equal to the rate of consumption of a reactant (or
formation of a product) divided by its coefficient in the balanced equation
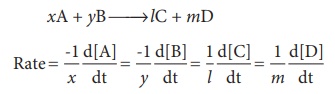
2. Average and instantaneous rate:
Let us understand the
average rate and instantaneous rate by considering the isomerisation of
cyclopropane.
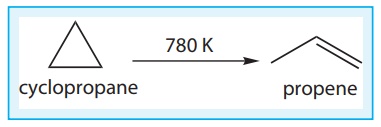
The kinetics of the
above reaction is followed by measuring the concentration of cyclopropane at
regular intervals and the observations are shown below. (Table 7.1)
Table 7.1 Concentration of cyclopropane at various times during its isomerisation at 780K
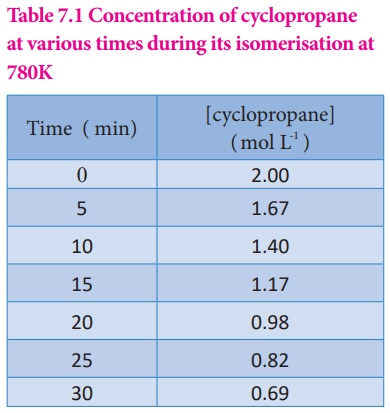
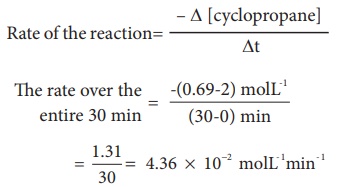
It means that during the
first 30 minutes of the reaction, the concentration of the reactant ( cyclo
propane) decreases as an average of 4.36 ×
10-2 mol L-1 each minute.
Let us calculate the
average rate for an initial and later stage over a short period.
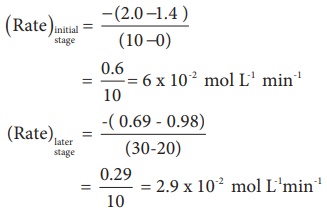
From the above
calculations, we come to know that the rate decreases with time as the reaction
proceeds and the average rate cannot be used to predict the rate of the
reaction at any instant. The rate of the reaction, at a particular instant
during the reaction is called the instantaneous rate. The shorter the time
period, we choose, the closer we approach to the instantaneous rate,
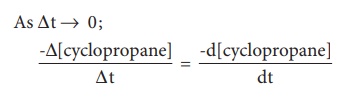
A plot of [cyclopropane]
Vs (time) gives a curve as shown in the figure 7.2. Instantaneous rate at a
particular instant ‘t ’ -d [cyclopropane] / dt the slope of a tangent drawn to
the curve at that instant.
In general, the
instantaneous reaction rate at a moment of mixing the reactants is calculated
from the slope of the tangent drawn to the curve at mol L-1 , the rate
calculated by this method is called initial rate of a reaction.
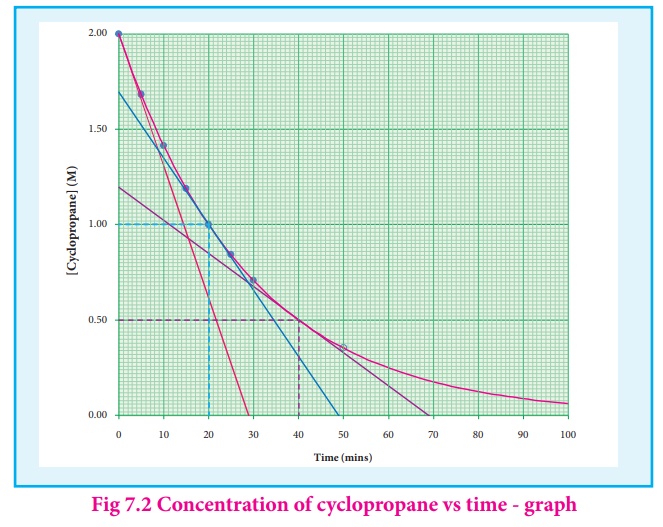
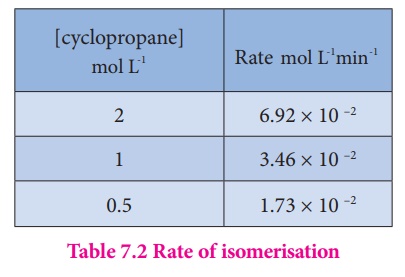
Let us calculate the
instantaneous rate of isomerisation cyclopropane at different concentrations: 2
M, 1M and 0.5 M from the graph shown in fig 7.2, the results obtained are
tabulated below.
3. Rate law and rate constant:
We have just learnt
that, the rate of the reaction depends upon the concentration of the reactant.
Now let us understand how the reaction rate is related to concentration by
considering the following general reaction.
xA + yB → products
The rate law for the
above reaction is generally expressed as
Rate = k [A]m
[B]n
Where k is
proportionality constant called the rate constant. The values of m and n
represent the reaction order with respect to A and B respectively. The overall
order of the reaction is given by (m+n). The values of the exponents (m and n)
in the rate law must be determined by experiment. They cannot be deduced from
the Stoichiometry of the reaction. For example, consider the isomerisation of
cyclopropane, that we discussed earlier.
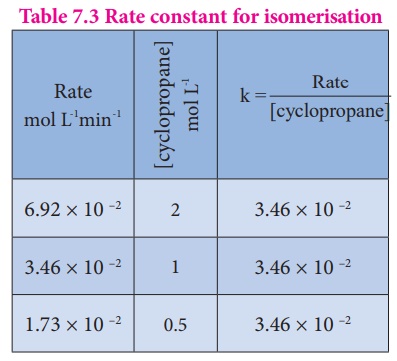
The results shown in
table 7.2 indicate that if the concentration of cyclopropane is reduced to
half, the rate also reduced to half. It means that the rate depends upon
[cyclopropane] raised to the first power
i.e., Rate =
k[cyclopropane]1
Rate /[cyclopropane] = k
Rate constant for isomerisation
Let us consider an
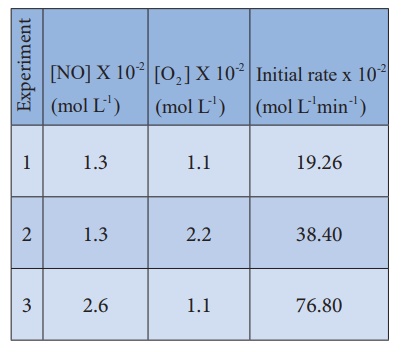
another example, the oxidation of nitric oxide (NO)
2NO(g) + O2 (g
) 2NO2 (g)
Series of experiments
are conducted by keeping the concentration of one of the reactants constant and
the changing the concentration of the others.
Rate = k [NO]m[O2]n
For experiment 1, the
rate law is
Rate1 = k
[NO]m[O2]n
19.26 X10-2 =
k [1.3] m[1.1]n ...(1)
Similarly for experiment
2
Rate2 = k
[NO]m [O2 ]n
38.40 X10-2 =
k [1.3] m[2.2]n ...(2)
For experiment 3

Therefore the reaction
is first order with respect to O2
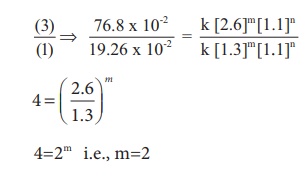
Therefore the reaction
is second order with respect to NO
The rate law is Rate1
= k [NO]2[O2 ]1
The overall order of the
reaction = (2 + 1) = 3
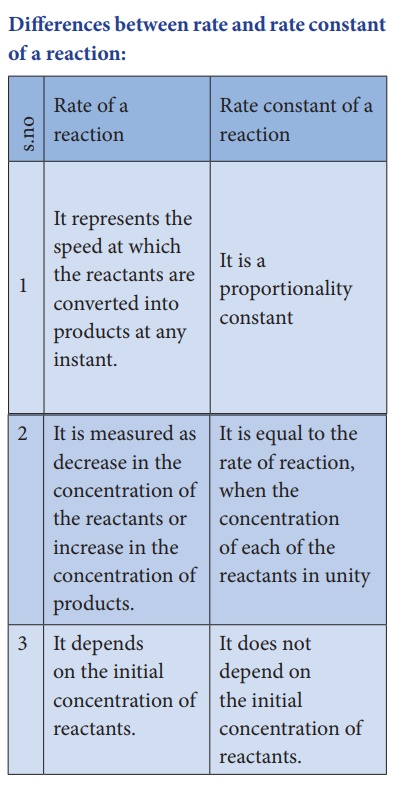
Differences between
rate and rate constant of a reaction:
Related Topics