Chapter: Modern Pharmacology with Clinical Applications: Pharmacological Management of Chronic Heart Failure
Quinidine
CLASS IA
Quinidine
Quinidine is an alkaloid
obtained from various species of Cinchona
or its hybrids, from Remijia pedunculata,
or from quinine. Quinidine is the dextrorotatory isomer of quinine .
Quinidine (Quinidex) was one of the first clinically
used antiarrhythmic agents. Because of the high inci-dence of ventricular
proarrhythmia associated with its use and numerous other equally efficacious
agents, quinidine is now used sparingly. Quinidine shares all of the
pharmacological properties of quinine, including an-timalarial, antipyretic,
oxytocic, and skeletal muscle re-laxant actions.
Electrophysiological Actions
Quinidine’s effect on the
electrical properties of a par-ticular cardiac tissue depends on the extent of
parasympathetic innervation, the level of parasympa-thetic tone, and the dose.
The anticholinergic actions of quinidine predominate at lower plasma
concentrations. Later, when steady-state therapeutic plasma concen-trations
have been achieved, the drug’s direct electro-physiological actions
predominate. The direct and indi-rect electrophysiological actions are
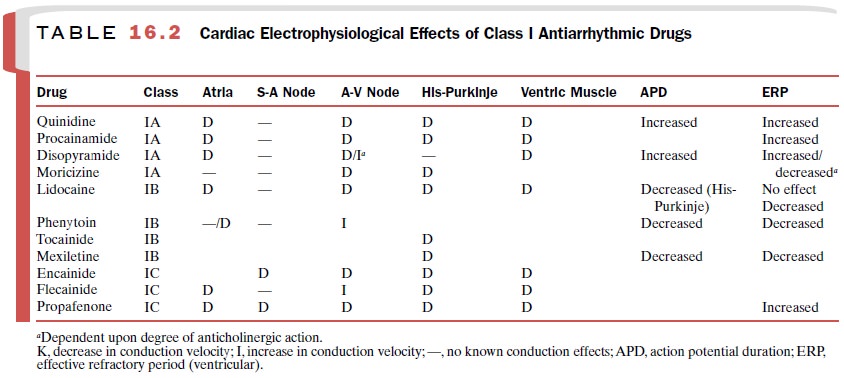
summarized in Table 16.2.
Sinoatrial Node and Atrial Tissue
The indirect effect of
quinidine on the sinoatrial node is a result of the drug’s potential to exert
an anti-cholinergic action resulting in a slight increase in heart rate. Higher
concentrations of quinidine have a direct effect of depressing the rate of
spontaneous diastolic depolarization.
Quinidine administration
results in a dose-depen-dent depression of membrane responsiveness in atrial
muscle fibers. The maximum rate of phase 0 depolariza-tion and the amplitude of
phase 0 are depressed equally at all membrane potentials. Quinidine also
decreases atrial muscle excitability in such a way that a larger cur-rent
stimulus is needed for initiation of an active re-sponse. These actions of
quinidine often are referred to as its local anesthetic properties.
A-V Node
Both the direct and indirect
actions of quinidine are important in determining its ultimate effect on A-V
conduction. The indirect (anticholinergic) properties of quinidine prevent both
vagally mediated prolongation of the A-V node refractory period and depression
of conduction velocity; these effects lead to enhancement of A-V transmission.
Quinidine’s direct electrophysio-logical actions on the A-V node are to
decrease con-duction velocity and increase the ERP.
His-Purkinje System and Ventricular Muscle
Quinidine can depress the
automaticity of ventricu-lar pacemakers by depressing the slope of phase 4
de-polarization. Depression of pacemakers in the His-Purkinje system is more
pronounced than depression of sinoatrial node pacemaker cells.
Quinidine also prolongs
repolarization in Purkinje fibers and ventricular muscle, increasing the duration
of the action potential. As in atrial muscle, quinidine ad-ministration results
in postrepolarization refractoriness, that is, an extension of refractoriness
beyond the recov-ery of the resting membrane potential. The indirect
(an-ticholinergic) properties of quinidine are not a factor in its actions on
ventricular muscle and the His-Purkinje system.
Serum K+ concentrations
have a major influence on the activity of quinidine on cardiac tissue. Low
extracel-lular K+ concentrations antagonize the depressant ef-fects
of quinidine on membrane responsiveness, whereas high extracellular K+ concentrations
increase quinidine’s ability to depress membrane responsive-ness. This
dependency may explain why hypokalemic patients are often unresponsive to the
antiarrhythmic effects of quinidine and are prone to develop cardiac rhythm
disorders.
Electrocardiographic Changes
At normal therapeutic plasma
concentrations, quinidine prolongs the PR, the QRS, and the QT intervals. QRS
and QT prolongations are more pronounced with quini-dine than with most other
antiarrhythmic agents. The magnitude of these changes is related directly to
the plasma quinidine concentration.
Hemodynamic Effects
Although myocardial
depression is not a problem in pa-tients with normal cardiac function, in
patients with compromised myocardial function, quinidine may de-press cardiac
contractility sufficiently to result in a de-crease in cardiac output, a
significant rise in left ventric-ular end-diastolic pressure, and overt heart
failure. Quinidine can relax vascular smooth muscle directly as well as
indirectly by inhibition of α1-adrenoceptors. The depressant effects of quinidine on the
cardiovascular system are most likely to occur after IV administration, and
therefore, quinidine should not be employed rou-tinely in the emergency
treatment of arrhythmias. Because of its potential to cause marked depression
of myocardial contractility and to decrease peripheral vas-cular resistance,
parenteral administration of quinidine is seldom indicated.
Pharmacokinetics
The pharmacokinetic
characteristics of quinidine:
Oral bioavailability : Almost
complete absorption
Onset of action : 1–3 hours
Peak response : 1–2 hours
Duration of action : 6–8
hours
Plasma half-life : 6 hours
Primary route of metabolism :
Hepatic; active metabolite
Primary route of excretion: 10–50%
renal (unchanged)
Therapeutic serum concentration:
2–4 μg /mL
Clinical Uses
Primary indications for the
use of quinidine include (1) abolition of premature complexes that have an
atrial, A-V junctional, or ventricular origin; (2) restoration of normal sinus
rhythm in atrial flutter and atrial fibrilla-tion after controlling the
ventricular rate with digitalis; (3) maintenance of normal sinus rhythm after
electrical conversion of atrial arrhythmias; (4) prophylaxis against
arrhythmias associated with electrical countershock; (5) termination of
ventricular tachycardia; and (6) suppres-sion of repetitive tachycardia
associated with Wolff-Parkinson-White (WPW) syndrome.
Although quinidine often is successful
in producing normal sinus rhythm, its administration in the presence of a rapid
atrial rate (flutter and possibly atrial fibrilla-tion) can lead to a further
and dangerous increase in the ventricular rate secondary to inhibition of basal
vagal tone upon the A-V node. For this reason, digitalis should be used
before quinidine when one is attempting to convert atrial flutter or atrial
fibrillation to normal si-nus rhythm.
Adverse Effects
The most common adverse
effects associated with quinidine administration are diarrhea (35%), upper
gastrointestinal distress (25%), and light-headedness (15%). Other relatively
common adverse effects in-clude fatigue, palpitations, headache (each occurring
with an incidence of 7%), anginalike pain, and rash. These adverse effects are
generally dose related and re-versible with cessation of therapy. In some
patients, quinidine administration may bring on thrombocytope-nia due to the
formation of a plasma protein–quinidine complex that evokes a circulating
antibody directed against the blood platelet. Although platelet counts re-turn
to normal on cessation of therapy, administration of quinidine or quinine at a
later date can cause the reappearance of thrombocytopenia.
The cardiac toxicity of
quinidine includes A-V and intraventricular block, ventricular
tachyarrhythmias, and depression of myocardial contractility. Ventricular
arrhythmia induced by quinidine leading to a loss of consciousness has been
referred to as quinidine syn-cope. This devastating side effect is more common
in women than in men and may occur at therapeutic or subtherapeutic plasma
concentrations.
Large doses of quinidine can
produce a syndrome known as cinchonism,
which is characterized by ringing in the ears, headache, nausea, visual
disturbances or blurred vision, disturbed auditory acuity, and vertigo. Larger
doses can produce confusion, delirium, hallucina-tions, or psychoses. Quinidine
can decrease blood glucose concentrations, possibly by inducing insulin
secretion.
Contraindications
One of the few absolute
contraindications for quinidine is complete A-V block with an A-V pacemaker or
id-ioventricular pacemaker; this may be suppressed by quinidine, leading to
cardiac arrest.
Persons with congenital QT
prolongation may de-velop torsades de pointes tachyarrhythmia and should not be
exposed to quinidine.
Owing to the negative
inotropic action of quinidine, it is contraindicated in congestive heart
failure and hy-potension.Digitalis intoxication and hyperkalemia can
accen-tuate the depression of conduction caused by quinidine.
Myasthenia gravis can be
aggravated severely by quinidine’s actions at the neuromuscular junction.
The use of quinidine and
quinine should be avoided in patients who previously showed evidence of
quini-dine-induced thrombocytopenia.
Drug Interactions
Quinidine can increase the
plasma concentrations of digoxin, which may in turn lead to signs and symptoms
of digitalis toxicity. Gastrointestinal, central nervous system (CNS), or
cardiac toxicity associated with elevated digoxin concentrations may occur.
Quinidine and digoxin can be administered concurrently; however, a downward
adjustment in the digoxin dose may be required.
Drugs that have been
associated with elevations in quinidine concentrations include acetazolamide,
the antacids magnesium hydroxide and calcium carbonate, and the H2-receptor
antagonist cimetidine. Cimetidine inhibits the hepatic metabolism of quinidine.
Phenytoin, rifampin, and barbiturates increase the hepatic metabo-lism of
quinidine and reduce its plasma concentrations.
Related Topics