Environmental Chemistry - Quality of drinking water | 11th Chemistry : UNIT 15 : Environmental Chemistry
Chapter: 11th Chemistry : UNIT 15 : Environmental Chemistry
Quality of drinking water
Quality
of drinking water:
Now
a days most of us hesitate to use natural water directly for drinking, because
biological, physical or chemical impurities from different sources mix with
surface water or ground water.
Institutions
like WHO (World Health Organisation) at world level and BIS (Bureau of Indian
Standards) and ICMR (ICMR: Indian Council of Medical Research) at national
level have prescribed standards for quality of drinking water. Standard
characteristics prescribed for deciding the quality of drinking water by BIS,
in 1991 are shown in Table.15.3
Table 15.3 Standard characteristics of drinking water
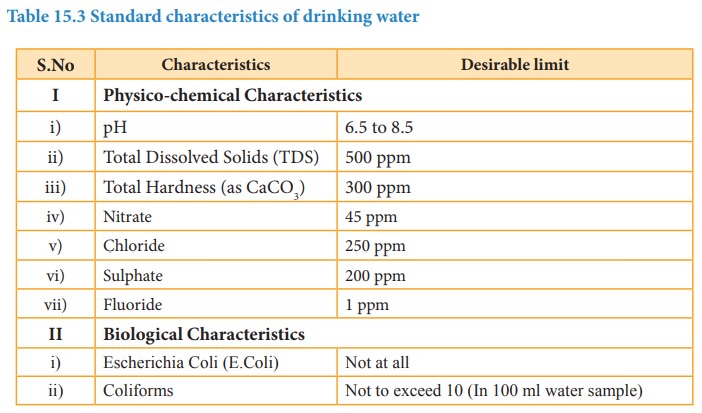
Characteristics and Desirable limit
I Physico-chemical Characteristics
i) pH : 6.5 to 8.5
ii) Total Dissolved Solids (TDS) : 500 ppm
iii) Total Hardness (as CaCO3) : 300 ppm
iv) Nitrate : 45 ppm
v) Chloride :
250 ppm
vi) Sulphate :
200 ppm
vii) Fluoride :
1 ppm
II Biological Characteristics
i) Escherichia Coli (E.Coli) : Not at all
ii) Coliforms : Not to exceed 10 (In 100 ml water
sample)
Fluoride:
Fluoride
ion deficiency in drinking water causes tooth decay. Water soluble fluorides
are added to increase the fluoride ion concentration upto 1 ppm.
The
Fluoride ions make the enamel on teeth much harder by converting
hydroxyapatite, [3(Ca3(PO4)2.Ca(OH)2],
the enamel on the surface of the teeth, into much harder fluorapatite, [3(Ca3(PO4)2.CaF2].
However,
Fluoride ion concentration above 2 ppm causes brown mottling of teeth.
Excess
fluoride causes damage to bone and teeth.
Lead :
Drinking
water containing lead contamination above 50ppb can cause damage to liver,
kidney and reproductive systems.
Sulphate:
Moderate
level of sulphate is harmless. Excessive concentration (>500ppm) of
sulphates in drinking water causes laxative effect.
Nitrate:
Use
of drinking water having concentration of nitrate higher than 45 ppm may causes
methemoglobinemia (blue baby syndrome) disease in children.
Total dissolved solids (TDS):
Most
of the salts are soluble in water. It includes cations like calcium, magnesium,
sodium, potassium, iron and anions like carbonate, bicarbonate, chloride,
sulphate, phosphate and nitrate. Use of drinking water having total dissolved
solids concentration higher than 500 ppm causes possibilities of irritation in
stomach and intestine.
Related Topics