Chapter: Basic & Clinical Pharmacology : Drugs Used in the Treatment of Gastrointestinal Diseases
Proton Pump Inhibitors
PROTON PUMP INHIBITORS
Since their introduction in the late 1980s,
these efficacious acid inhibitory agents have assumed the major role for the
treatment of acid-peptic disorders. Proton pump inhibitors (PPIs) are now among
the most widely prescribed drugs worldwide due to their outstanding efficacy
and safety.
Chemistry & Pharmacokinetics
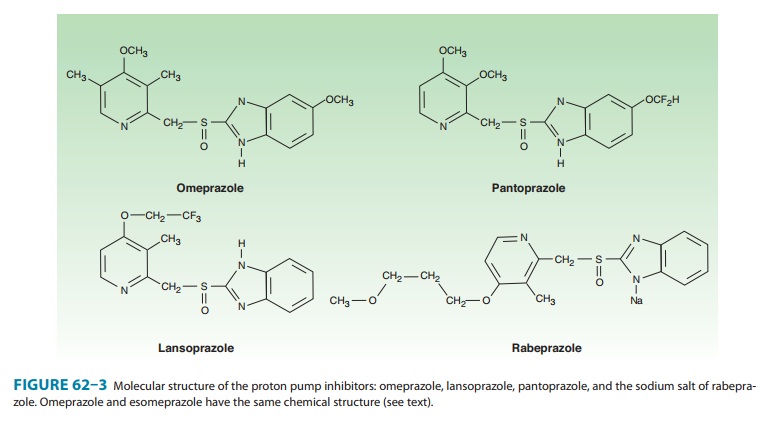
Six proton pump inhibitors are available for
clinical use: omepra-zole, esomeprazole,
lansoprazole, dexlansoprazole, rabepra-zole, and pantoprazole. All are substituted benzimidazoles thatresemble H2 antagonists in
structure (Figure 62–3) but have a completely different mechanism of action.
Omeprazole and lanso-prazole are racemic mixtures of R- and S-isomers.
Esomeprazole is the S-isomer of
omeprazole and dexlansoprazole the R-isomer
of lansoprazole. All are available in oral formulations. Esomeprazole and
pantoprazole are also available in intravenous formulations (Table 62–2).
Proton pump inhibitors are administered as
inactive prodrugs. To protect the acid-labile prodrug from rapid destruction
within the gastric lumen, oral products are formulated for delayed release as
acid-resistant, enteric-coated capsules or tablets. After passing through the
stomach into the alkaline intestinal lumen, the enteric
For children or patients with dysphagia
or enteral feeding tubes, capsule formula-tions (but not tablets) may be opened
and the microgranules mixed with apple or orange juice or mixed with soft foods
(eg, applesauce). Lansoprazole is also available as a tablet formulation that
disintegrates in the mouth, or it may be mixed with water and administered via
oral syringe or enteral tube. Omeprazole is also available as a powder
formulation (capsule or packet) that contains sodium bicarbonate (1100–1680 mg
NaHCO3; 304–460 mg of sodium) to protect
the naked (non-enteric-coated) drug from acid degradation. When administered on
an empty stomach by mouth or enteral tube, this “immediate-release” suspension
results in rapid omeprazole absorption (Tmax< 30
minutes) and onset of acid inhibition.
The proton
pump inhibitors are lipophilic weak bases (pKa
4–5) and after intestinal absorption diffuse readily across lipid membranes into
acidified compartments (eg, the parietal cell canaliculus). The prodrug rapidly
becomes protonated within the canaliculus and is concentrated more than
1000-fold by Henderson-Hasselbalch trapping . There, it rapidly undergoes a
molecular conversion to the active form, a reactive thiophilic sulfenamide
cation, which forms a covalent disulfide bond with the H+/K+-ATPase, irreversibly
inactivating the enzyme.
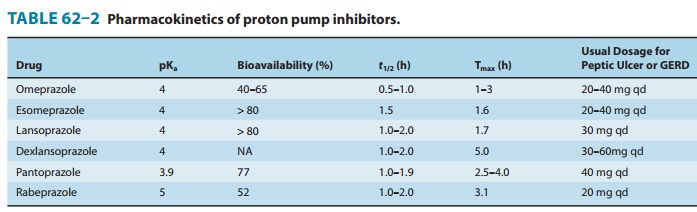
The pharmacokinetics of available proton pump inhibitors are shown in Table 62–2. Immediate-release omeprazole has a faster onset of acid inhibition than other oral formulations. Although differences in pharmacokinetic profiles may affect speed of onset and duration of acid inhibition in the first few days of therapy, they are of little clinical importance with continued daily admin-istration. The bioavailability of all agents is decreased approxi-mately 50% by food; hence, the drugs should be administered on an empty stomach.
In a fasting state, only 10%
of proton pumps are actively secreting acid and susceptible to inhibition.
Proton pump inhibitors should be administered approximately 1 hour before a
meal (usually breakfast), so that the peak serum concen-tration coincides with
the maximal activity of proton pump secre-tion. The drugs have a short serum
half-life of about 1.5 hours, but acid inhibition lasts up to 24 hours owing to
the irreversible inactivation of the proton pump. At least 18 hours are
required for synthesis of new H+/K+-ATPase pump
molecules. Because not all proton pumps are inactivated with the first dose of
medication, up to 3–4 days of daily medication are required before the full
acid-inhibiting potential is reached. Similarly, after stopping the drug, it
takes 3–4 days for full acid secretion to return.
Proton pump inhibitors undergo rapid
first-pass and systemic hepatic metabolism and have negligible renal clearance.
Dose reduction is not needed for patients with renal insufficiency or mild to
moderate liver disease but should be considered in patients with severe liver
impairment. Although other proton pumps exist in the body, the H+/K+-ATPase appears to exist only in the parietal cell and is distinct
structurally and functionally from other H+-transporting enzymes.
The intravenous formulations of esomeprazole
and pantopra-zole have characteristics similar to those of the oral drugs. When
given to a fasting patient, they inactivate acid pumps that are actively
secreting, but they have no effect on pumps in quiescent, nonsecreting
vesicles. Because the half-life of a single injection of the intravenous
formulation is short, acid secretion returns several hours later as pumps move
from the tubulovesicles to the canali-cular surface. Thus, to provide maximal
inhibition during the first 24–48 hours of treatment, the intravenous
formulations must be given as a continuous infusion or as repeated bolus
injections. The optimal dosing of intravenous proton pump inhibitors to achieve
maximal blockade in fasting patients is not yet established.
From a pharmacokinetic perspective, proton
pump inhibitors are ideal drugs: they have a short serum half-life, they are
concen-trated and activated near their site of action, and they have a long
duration of action.
Pharmacodynamics
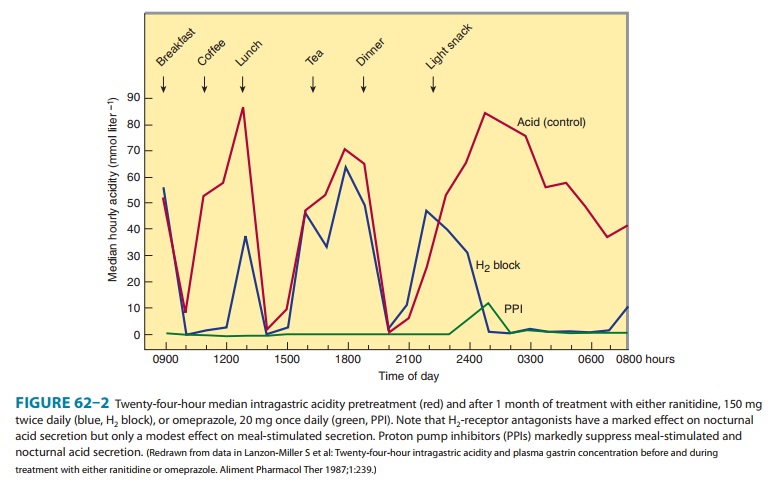
In contrast to H2 antagonists, proton pump inhibitors inhibit both fasting and meal-stimulated secretion because they block the final common pathway of acid secretion, the proton pump. In standard doses, proton pump inhibitors inhibit 90–98% of 24-hour acid secretion (Figure 62–2). When administered at equivalent doses, the different agents show little difference in clinical efficacy. In a crossover study of patients receiving long-term therapy with five proton pump inhibitors, the mean 24-hour intragastric pH varied from 3.3 (pantoprazole, 40 mg) to 4.0 (esomeprazole, 40 mg) and the mean number of hours the pH was higher than 4 varied from 10.1 (pantoprazole, 40 mg) to 14.0 (esomeprazole, 40 mg). Although dexlansoprazole has a delayed-release formulation that results in a longer Tmax and greater AUC than other proton pump inhibitors, it appears comparable to other agents in the ability to suppress acid secretion. This is because acid suppression is more dependent upon irreversible nactivation of the proton pump than the pharmacokinetics of different agents.
Clinical Uses
A. Gastroesophageal Reflux Disease
(GERD)
Proton pump inhibitors are the most effective
agents for the treat-ment of nonerosive and erosive reflux disease, esophageal
compli-cations of reflux disease (peptic stricture or Barrett’s esophagus), and
extraesophageal manifestations of reflux disease. Once-daily dosing provides
effective symptom relief and tissue healing in 85–90% of patients; up to 15% of
patients require twice-daily dosing.
GERD
symptoms recur in over 80% of patients within 6 months after discontinuation of
a proton pump inhibitor. For patients with erosive esophagitis or esophageal
complications, long-term daily maintenance therapy with a full-dose or
half-dose proton pump inhibitor is usually needed. Many patients with
nonerosive GERD may be treated successfully with intermittent courses of proton
pump inhibitors or H2
antagonists taken as needed (“on demand”) for recurrent symptoms.
In current
clinical practice, many patients with symptomatic GERD are treated empirically
with medications without prior endoscopy, ie, without knowledge of whether the
patient has ero-sive or nonerosive reflux disease. Empiric treatment with
proton pump inhibitors provides sustained symptomatic relief in 70–80% of
patients, compared with 50–60% with H2
antagonists. Because of recent cost reductions, proton pump inhibitors are
being used increasingly as first-line therapy for patients with symptomatic
GERD.
Sustained acid suppression with twice-daily
proton pump inhibitors for at least 3 months is used to treat extraesophageal
complications of reflux disease (asthma, chronic cough, laryngitis, and
noncardiac chest pain).
B. Peptic Ulcer Disease
Compared
with H2 antagonists, proton pump
inhibitors afford more rapid symptom relief and faster ulcer healing for
duodenal ulcers and, to a lesser extent, gastric ulcers. All the pump
inhibi-tors heal more than 90% of duodenal ulcers within 4 weeks and a similar
percentage of gastric ulcers within 6–8 weeks.
1.H
pylori-associated ulcers— ForH pylori-associated ulcers,there are two therapeutic goals: to heal
the ulcer and to eradicate the organism. The most effective regimens for H pylori eradication are combinations of
two antibiotics and a proton pump inhibitor. Proton pump inhibitors promote
eradication of H pylori through
several mechanisms: direct antimicrobial properties (minor) and—by raising
intragastric pH—lowering the minimal inhibi-tory concentrations of antibiotics
against H pylori. The best treat-ment
regimen consists of a 14-day regimen of “triple therapy”: a proton pump
inhibitor twice daily; clarithromycin, 500 mg twice daily; and either
amoxicillin, 1 g twice daily, or metronidazole,mg twice daily. After completion
of triple therapy, the proton pump inhibitor should be continued once daily for
a total of 4–6 weeks to ensure complete ulcer healing. Alternatively, 10 daysof
“sequential treatment” consisting on days 1–5 of a proton pump inhibitor twice
daily plus amoxicillin, 1 g twice daily, and followed on days 6–10 by five
additional days of a proton pump inhibitor twice daily, plus clarithromycin,
500 mg twice daily, and tinidazole, 500 mg twice daily, has been shown to be a
highly effective treatment regimen.
2. NSAID-associated
ulcers—For
patients with ulcers causedby aspirin or other NSAIDs, either H2 antagonists or proton
pump inhibitors provide rapid ulcer healing so long as the NSAID is
discontinued; however continued use of the NSAID impairs ulcer healing. In
patients with NSAID-induced ulcers who require continued NSAID therapy,
treatment with a once- or twice-daily proton pump inhibitor more reliably
promotes ulcer healing.
Asymptomatic peptic ulceration develops in
10–20% of people taking frequent NSAIDs, and ulcer-related complications
(bleed-ing, perforation) develop in 1–2% of persons per year. Proton pump
inhibitors taken once daily are effective in reducing the incidence of ulcers
and ulcer complications in patients taking aspirin or other NSAIDs.
3.
Prevention of rebleeding from peptic ulcers—In patientswith acute gastrointestinal
bleeding due to peptic ulcers, the risk of rebleeding from ulcers that have a
visible vessel or adherent clot is increased. Rebleeding of this subset of
high-risk ulcers is reduced significantly with proton pump inhibitors
administered for 3–5 days either as high-dose oral therapy (eg, omeprazole, 40
mg orally twice daily) or as a continuous intravenous infusion. It is believed
that an intragastric pH higher than 6 may enhance coagulation and platelet
aggregation. The optimal dose of intravenous proton pump inhibitor needed to
achieve and maintain this level of near-complete acid inhibition is unknown;
however, initial bolus administration of esomeprazole or pantoprazole (80 mg)
followed by constant infusion (8 mg/h) is commonly recommended.
C. Nonulcer Dyspepsia
Proton pump inhibitors have modest efficacy
for treatment of non-ulcer dyspepsia, benefiting 10–20% more patients than
placebo. Despite their use for this indication, superiority to H2 antagonists (or even
placebo) has not been conclusively demonstrated.
D. Prevention of Stress-Related Mucosal Bleeding
As
discussed previously (see H2-Receptor
Antagonists) proton pump inhibitors (given orally, by nasogastric tube, or by
intrave-nous infusions) may be administered to reduce the risk of clini-cally
significant stress-related mucosal bleeding in critically ill patients. The
only proton pump inhibitor approved by the Food and Drug Administration (FDA)
for this indication is an oral immediate-release omeprazole formulation, which
is administered by nasogastric tube twice daily on the first day, then once
daily. For patients with nasoenteric tubes, immediate-release omeprazole may be
preferred to intravenous H2
antagonists or other proton pump inhibitors because of comparable efficacy,
lower cost, and ease of administration.
For patients without a nasoenteric tube or
with significant ileus, intravenous H2 antagonists are preferred to intravenous
proton pump inhibitors because of their proven efficacy and lower cost.
Although proton pump inhibitors are increasingly used, there are no controlled
trials demonstrating efficacy or optimal dosing.
E. Gastrinoma and Other Hypersecretory Conditions
Patients with isolated gastrinomas are best
treated with surgical resection. In patients with metastatic or unresectable
gastrinomas, massive acid hypersecretion results in peptic ulceration, erosive
esophagitis, and malabsorption. Previously, these patients required vagotomy
and extraordinarily high doses of H2 antagonists, which still resulted in
suboptimal acid suppression. With proton pump inhibitors, excellent acid
suppression can be achieved in all patients. Dosage is titrated to reduce basal
acid output to less than 5–10 mEq/h. Typical doses of omeprazole are 60–120
mg/d.
Adverse Effects
A. General
Proton pump inhibitors are extremely safe.
Diarrhea, headache, and abdominal pain are reported in 1–5% of patients,
although the frequency of these events is only slightly increased compared with
placebo. Increasing cases of acute interstitial nephritis have been reported.
Proton pump inhibitors do not have teratogenicity in animal models; however,
safety during pregnancy has not been established.
B. Nutrition
Acid is
important in releasing vitamin B12
from food. A minor reduction in oral cyanocobalamin absorption occurs during
pro-ton pump inhibition, potentially leading to subnormal B12
levels with prolonged therapy. Acid also promotes absorption of food-bound
minerals (non-heme iron, insoluble calcium salts, magne-sium). Several
case-control studies have suggested a modest increase in the risk of hip
fracture in patients taking proton pump inhibitors over a long term compared
with matched controls. Although a causal relationship is unproven, proton pump
inhibi-tors may reduce calcium absorption or inhibit osteoclast function.
Pending further studies, patients who require long-term proton pump
inhibitors—especially those with risk factors for osteoporo-sis—should have
monitoring of bone density and should be pro-vided calcium supplements. Cases
of severe, life-threatening hypomagnesemia with secondary hypocalcemia due to
proton pump inhibitors have been reported; however, the mechanism of action is
unknown.
C. Respiratory and Enteric Infections
Gastric
acid is an important barrier to colonization and infection of the stomach and
intestine from ingested bacteria. Increases in gastric bacterial concentrations
are detected in patients taking proton pump inhibitors, which is of unknown
clinical signifi-cance. Some studies have reported an increased risk of both
com-munity-acquired respiratory infections and nosocomial pneumonia among
patients taking proton pump inhibitors.
There is a
2- to 3-fold increased risk for hospital- and community-acquired Clostridium difficile infection in
patients taking proton pump inhibitors. There also is a small increased risk of
other enteric infections (eg, Salmonella,
Shigella, E coli, Campylobacter), which should be considered particularly
when traveling in under-developed countries.
D. Potential Problems Due to Increased Serum Gastrin
Gastrin levels are regulated by intragastric
acidity. Acid suppres-sion alters normal feedback inhibition so that median
serum gas-trin levels rise 1.5- to 2-fold in patients taking proton pump
inhibitors. Although gastrin levels remain within normal limits in most
patients, they exceed 500 pg/mL (normal, < 100 pg/mL) in 3%.
Upon stopping the drug, the levels normalize within 4 weeks. The rise in serum
gastrin levels may stimulate hyperplasia of ECL and parietal cells, which may
cause transient rebound acid hyper-secretion with increased dyspepsia or
heartburn after drug discon-tinuation, which abate within 2–4 weeks after
gastrin and acid secretion normalize. In female rats given proton pump
inhibitors for prolonged periods, hypergastrinemia caused gastric carcinoid
tumors that developed in areas of ECL hyperplasia. Although humans who take
proton pump inhibitors for a long time also may exhibit ECL hyperplasia,
carcinoid tumor formation has not been documented. At present, routine
monitoring of serum gas-trin levels is not recommended in patients receiving
prolonged proton pump inhibitor therapy.
E. Other Potential Problems Due to Decreased Gastric Acidity
Among patients infected with H pylori, long-term acid suppres-sion
leads to increased chronic inflammation in the gastric body and decreased
inflammation in the antrum. Concerns have been raised that increased gastric
inflammation may accelerate gastric gland atrophy (atrophic gastritis) and intestinal
metaplasia— known risk factors for gastric adenocarcinoma. A special FDA
Gastrointestinal Advisory Committee concluded that there is no evidence that
prolonged proton pump inhibitor therapy produces the kind of atrophic gastritis
(multifocal atrophic gastritis) or intestinal metaplasia that is associated
with increased risk of ade-nocarcinoma. Routine testing for H pylori is not recommended in patients
who require long-term proton pump inhibitor therapy. Long-term proton pump
inhibitor therapy is associated with the development of small benign gastric
fundic-gland polyps in a small number of patients, which may disappear after
stopping the drug and are of uncertain clinical significance.
Drug Interactions
Decreased gastric acidity may alter absorption
of drugs for which intragastric acidity affects drug bioavailability, eg,
ketoconazole, itraconazole, digoxin, and atazanavir. All proton pump inhibitors
are metabolized by hepatic P450 cytochromes, including CYP2C19 and CYP3A4.
Because of the short half-lives of proton pump inhibitors, clinically
significant drug interactions are rare. Omeprazole may inhibit the metabolism
of warfarin, diazepam, and phenytoin. Esomeprazole also may decrease metabolism
ofdiazepam. Lansoprazole may enhance clearance of theophyl-line. Rabeprazole
and pantoprazole have no significant drug interactions.
The FDA has
issued a warning about a potentially important adverse interaction between
clopidogrel and proton pump inhibi-tors. Clopidogrel is a prodrug that requires
activation by the hepatic P450 CYP2C19 isoenzyme, which also is involved to
varying degrees in the metabolism of proton pump inhibitors (especially
omeprazole, esomeprazole, lansoprazole, and dexlanso-prazole). Thus, proton
pump inhibitors could reduce clopidogrel activation (and its antiplatelet
action) in some patients. Several large retrospective studies have reported an
increased incidence of serious cardiovascular events in patients taking
clopidogrel and a proton pump inhibitor. In contrast, three smaller prospective
randomized trials have not detected an increased risk. Pending further studies,
proton pump inhibitors should be prescribed to patients taking clopidogrel only
if they have an increased risk of gastrointestinal bleeding or require them for
chronic gastro-esophageal reflux or peptic ulcer disease, in which case agents
with minimal CYP2C19 inhibition (pantoprazole or rabeprazole) are preferred.
Related Topics