Chapter: Medical Physiology: Regulation of Acid-Base Balance
Proteins: Important Intracellular Buffers
Proteins: Important Intracellular Buffers
Proteins are among the most plentiful buffers in the body because of their high concentrations, especially within the cells.
The pH of the cells, although slightly lower than in the extracellular fluid, nevertheless changes approxi-mately in proportion to extracellular fluid pH changes. There is a slight amount of diffusion of H+ and HCO3– through the cell membrane, although these ions require several hours to come to equilibrium with the extracellular fluid, except for rapid equilibrium that occurs in the red blood cells. CO2, however, can rapidly diffuse through all the cell membranes. This diffusionof the elements of the bicarbonate buffer system causes the pH in intracellular fluid to change when there are changes in extracellular pH. For this reason, the buffersystems within the cells help prevent changes in the pH of extracellular fluid but may take several hours to become maximally effective.
In the red blood cell, hemoglobin (Hb) is an impor-tant buffer, as follows:
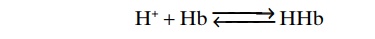
Approximately 60 to 70 per cent of the total chemi-cal buffering of the body fluids is inside the cells, and most of this results from the intracellular proteins.
However, except for the red blood cells, the slowness with which H+ and HCO3– move through the cell mem-branes often delays for several hours the maximum ability of the intracellular proteins to buffer extracel-lular acid-base abnormalities.
In addition to the high concentration of proteins in the cells, another factor that contributes to their buffering power is the fact that the pKs of many of these protein systems are fairly close to 7.4.
Isohydric Principle: All Buffers in a Common Solution Are in Equilibrium with the Same Hydrogen Ion Concentration
We have been discussing buffer systems as though they operated individually in the body fluids. However, they all work together, because H+ is common to the reac-tions of all the systems. Therefore, whenever there is a change in H+ concentration in the extracellular fluid, the balance of all the buffer systems changes at the same time. This phenomenon is called the isohydric principleand is illustrated by the following formula:
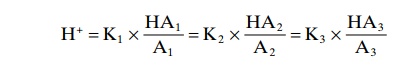
K1, K2, K3 are the dissociation constants of three respective acids, HA1, HA2, HA3, and A1, A2, A3 are the concentrations of the free negative ions that constitute the bases of the three buffer systems.
The implication of this principle is that any condition that changes the balance of one of the buffer systems also changes the balance of all the others because the buffer systems actually buffer one another by shifting H+ back and forth between them.
Related Topics