Chapter: Modern Analytical Chemistry: Evaluating Analytical Data
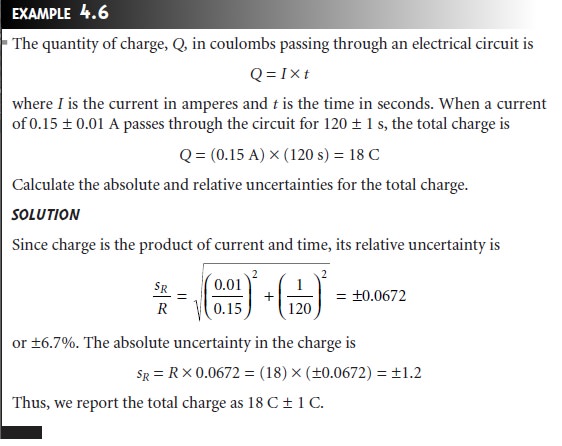
Propagation of Uncertainty
Propagation of
Uncertainty
Suppose that you
need to add
a reagent to a flask
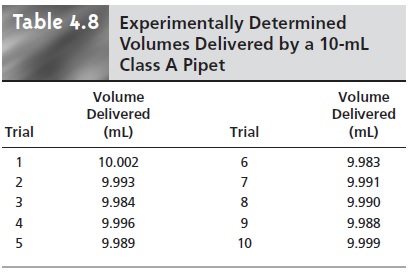
by several successive transfers using a class A 10-mL pipet. By calibrating the pipet (see Table 4.8), you know that
it delivers a volume of 9.992 mL with a standard deviation of 0.006 mL.
Since the pipet is calibrated, we can use the standard
deviation as a measure of uncertainty.
This uncertainty tells
us that when
we use the
pipet to repetitively deliver 10 mL of
solution, the volumes
actually delivered are
randomly scattered around
the mean of 9.992 mL.
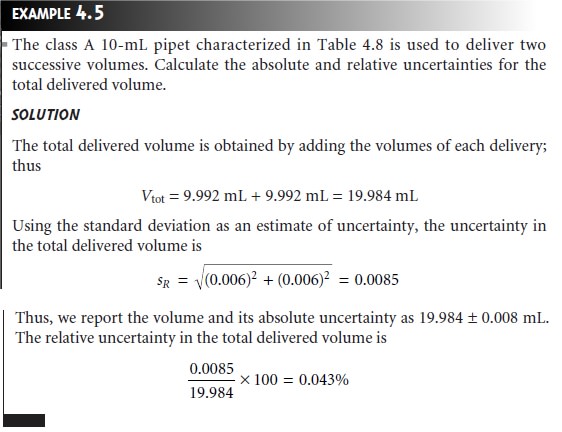
If the uncertainty in using the
pipet once is 9.992 ± 0.006 mL,
what is the
un- certainty when the pipet is used twice?
As a first guess, we might simply
add the un- certainties for each delivery; thus
(9.992 mL + 9.992 mL) ± (0.006 mL +
0.006 mL) = 19.984 ± 0.012 mL
It is easy
to see that
combining uncertainties in this way
overestimates the total
un- certainty. Adding the uncertainty for the first delivery to that of the second delivery
assumes that both volumes are
either greater than
9.992 mL or less than
9.992 mL. At the other extreme,
we might assume
that the two deliveries will always be on op- posite sides of the pipet’s mean volume. In this case we subtract
the uncertainties for the two deliveries,
(9.992 mL + 9.992 mL) ± (0.006 mL – 0.006 mL) = 19.984 ± 0.000
mL
underestimating the total uncertainty.
So what is the total
uncertainty when using
this pipet to deliver two successive
volumes of solution? From the
previous discussion we know that
the total uncer- tainty is greater than ±0.000 mL and less than ±0.012 mL. To estimate the cumula-
tive effect of multiple uncertainties, we use a mathematical technique known as the propagation of uncertainty. Our treatment of the propagation of uncertainty is based
on a few simple rules
that we will
not derive. A more thorough treatment can be
found elsewhere.
A Few Symbols
Propagation of uncertainty allows us to estimate the uncertainty in a calculated re- sult from the uncertainties of the measurements used to calculate the result. In the
equations presented in this section
the result is represented by the symbol
R
and the measurements by the symbols
A, B, and C. The
corresponding uncertainties are sR, sA, sB, and sC. The uncertainties for
A, B, and C can
be reported in several ways,
in- cluding calculated standard
deviations or estimated ranges, as long as the same form is
used for all measurements.
Uncertainty When Adding or Subtracting
When measurements are added
or subtracted, the
absolute uncertainty in the result is the square root of the sum of the squares
of the absolute uncertainties for the in- dividual measurements. Thus, for the equations R = A + B +
C
or R = A +
B
– C, or any other combination of adding and subtracting A, B, and C, the
absolute uncer- tainty in R is
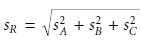 4.6
4.6
Uncertainty When Multiplying or Dividing
When measurements are multiplied or divided, the relative uncertainty in the result is the square root of the sum of the squares of the relative uncertainties for the indi- vidual measurements. Thus, for the equations R = A x B x C or R = A x B/C, or any other combination of multiplying and dividing A, B, and C, the relative uncertainty in R is
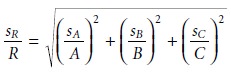
Uncertainty for Mixed Operations
Many chemical calculations involve a combination of adding and subtracting, and multiply and dividing. As shown in the following example, the propagation of un- certainty is easily calculated by treating each operation separately using equations 4.6
and 4.7 as needed.
Uncertainty for Other Mathematical Functions
Many other mathematical operations are commonly
used in analytical chemistry, including powers, roots, and logarithms. Equations
for the propagation of uncer- tainty for some of these
functions are shown
in Table 4.9.
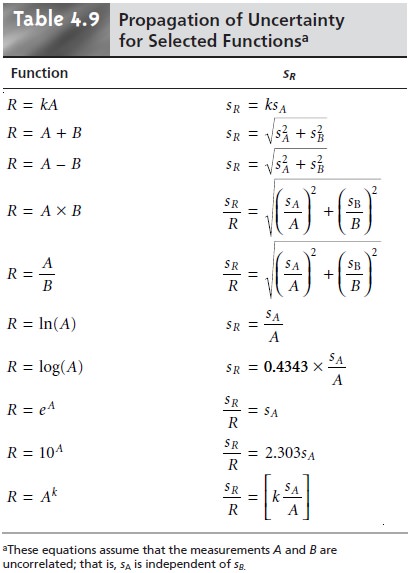
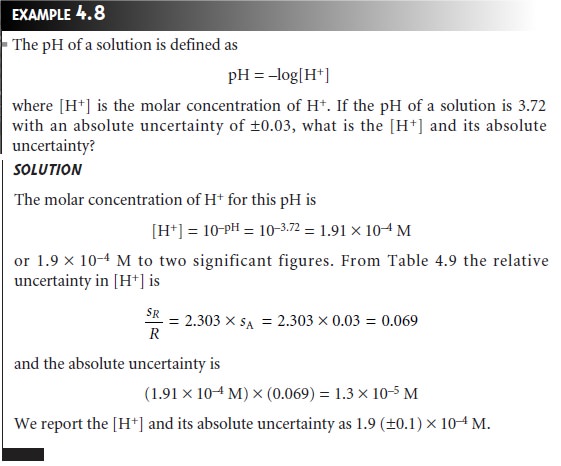
Is Calculating Uncertainty Actually Useful?
Given
the complexity
of determining a result’s uncertainty when several mea- surements are involved, it is worth
examining some of the reasons
why such cal- culations are useful. A propagation of uncertainty allows us to estimate an ex- pected
uncertainty for an analysis. Comparing the expected uncertainty to that which is actually obtained
can provide useful information. For example, in de- termining the mass of a penny,
we estimated the uncertainty in measuring mass as
±0.002 g based
on the balance’s tolerance. If we measure
a single penny’s
mass several times and
obtain a standard deviation of ±0.020
g, we would have reason
to believe that our measurement process is out of control.
We would then try to identify and correct the problem.
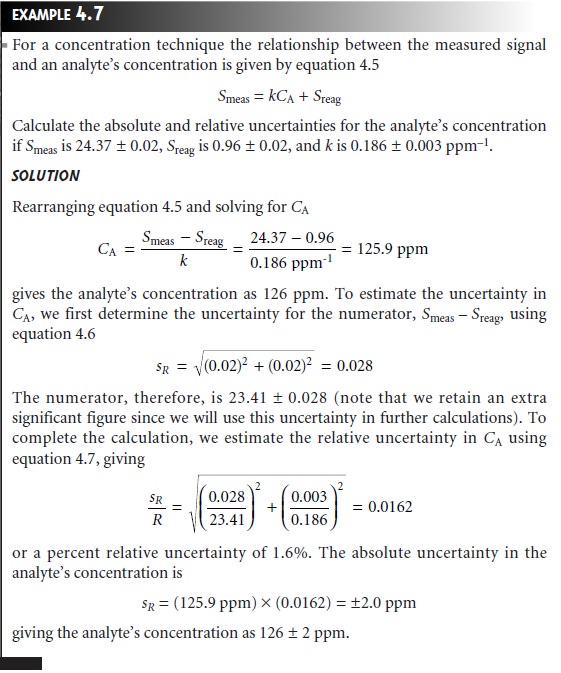
A propagation of uncertainty also
helps in deciding how to improve
the un- certainty in an analysis. In Example 4.7,
for instance, we calculated the
concen- tration of an analyte, obtaining a value of 126 ppm with an absolute uncertainty of ±2 ppm and a relative
uncertainty of 1.6%.
How might we improve the analy- sis so that the absolute uncertainty is only ±1 ppm (a relative uncertainty of 0.8%)? Looking
back on the calculation, we find that the relative
uncertainty is determined by the relative
uncertainty in the measured signal
(corrected for the reagent blank)
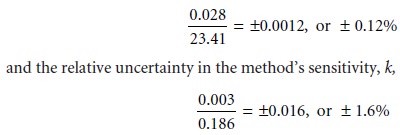
Of these two terms, the sensitivity’s uncertainty dominates the
total uncertainty. Measuring the signal more carefully will not improve the
overall uncertainty of the analysis. On the other hand, the desired improvement
in uncertainty can be achieved if the sensitivity’s absolute uncertainty can be
decreased to ±0.0015 ppm–1.
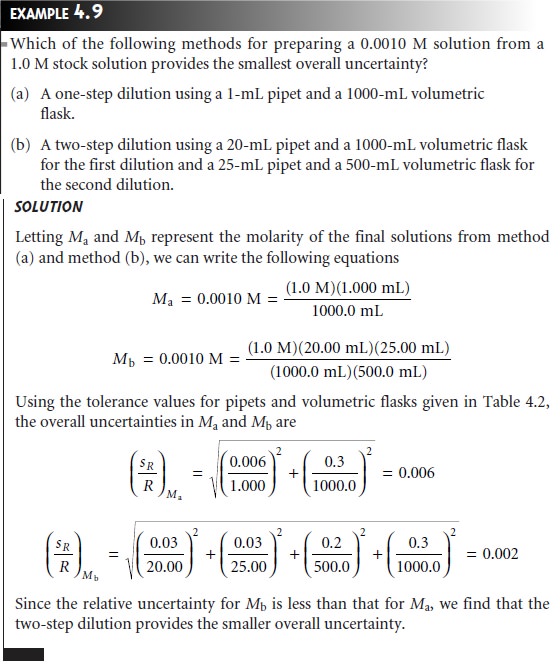
As a final
example, a propagation of uncertainty can be used to decide
which of several procedures provides the smallest overall uncertainty.
Preparing a solu- tion by diluting a stock solution
can be done using several
different combina- tions of volumetric glassware. For instance, we can dilute
a solution by a factor
of 10 using a 10-mL
pipet and a 100-mL volumetric flask, or by using a 25-mL
pipet and a 250-mL volumetric
flask. The same dilution also can be accom- plished in two steps
using a 50-mL
pipet and a 100-mL volumetric flask for the first dilution, and a 10-mL
pipet and a 50-mL volumetric flask for the second di- lution.
The overall uncertainty, of course, depends on the uncertainty of the glassware used
in the dilutions. As shown in the following example, we can
use the tolerance values for volumetric glassware to determine the optimum dilution strategy.
Related Topics