Chapter: Physics : Photonics and fibre Optics
Principle of Spontaneous and Stimulated emission - Einstein’s Quantum theory of radiation
Principle of Spontaneous and Stimulated emission – Einstein’s Quantum theory ofradiation
We know that, when light is absorbed by the atoms or molecules, then it goes from the lower energy level (E1) to the higher energy level (E2) and during the transition from higher energy level (E2) to lower energy level (E1) the light is emitted from the atoms or molecules.
Let us consider an atom exposed to light photons of energy E2 -E1= hv , three distinct processes take place.
a. Absorption
b.Spontaneous emission
c.Stimulated Emission
Absorption:
An atom in the lower energy level or ground state energy level E1 absorbs the incident photon radiation of energy hv and goes to the higher energy level or excited level E2 as shown in figure.
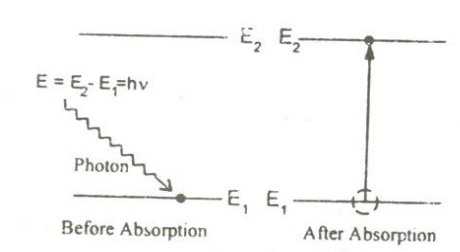
This process is called absorption
If there are many numbers of atoms in the ground state then each atom will absorb the energy from the incident photon and goes to the excited state. then,
The rate of absorption (R12) is proportional to the following factors.

Where B12 is a constant which gives the probability of absorption of absorption transition per unit time.
Normally, the atoms in the excited state will not stay there for a long period of time , rather it comes to ground state by emitting a photon of energy E=hν Such an emission takes place by one of the following two methods.
Spontaneous emission:
The atom in the excited state returns to the ground state by emitting a photon of energy
E=(E2-E1)=hν
Spontaneously without any external triggering as shown in the figure.
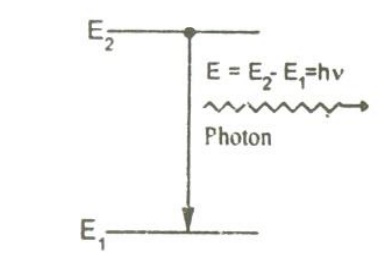
This process is known as spontaneous emission. Such an emission is random and is independent of incident radiation. If N1 and N2are the numbers of atoms in the ground state (E1) and excited state (E2) respectively, then
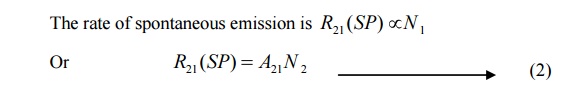
Where A21 is a constant which gives the probability of spontaneous emission transitions per unit time.
Stimulated Emission:
The atom in the excited state can also return to the ground state by external triggering or inducement of photon thereby emitting a photon of energy equal to the energy of the incident photon, known as stimulated emission. Thus results in two photons of same energy, phase difference and of same directionality as shown.
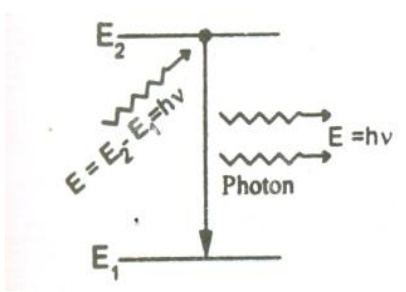
Therefore, the rate of stimulated emission is given by
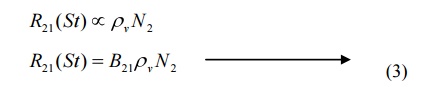
Where B21 is a constant which gives the probability of stimulated emission transitions per unit time.
Einstein’s theory
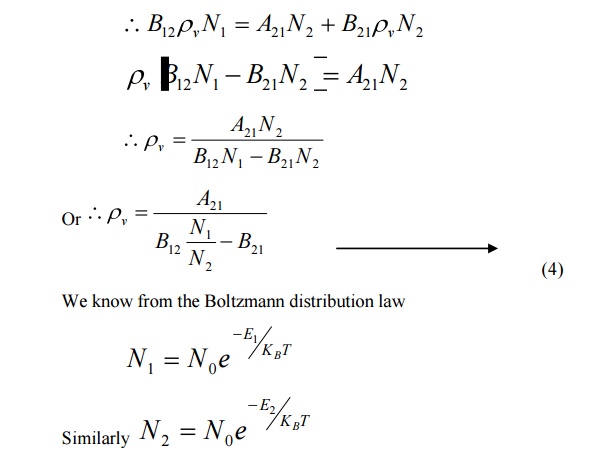
Einstein’s theory of absorption and emission of light by an atom is based on Planck’s theory of radiation. Also under thermal equilibrium, the population of energy levels obeys the Boltzmann distribution law
Under thermal equilibrium
The rate of absorption = the rate of emission
Where KB is the Boltzmann Constant,
T is the absolute temperature and
N0 is the number of atoms at absolute zero.
At equilibrium, we can write the ratio of population levels as follows.
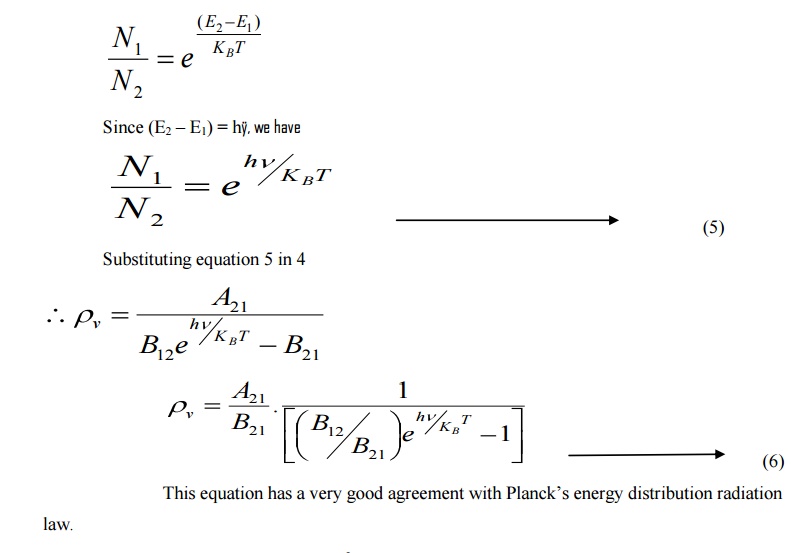
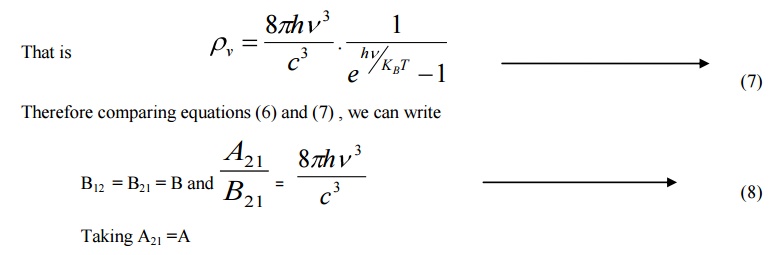
The constants A and B are called as Einstein Coefficients, which accounts for spontaneous and stimulated emission probabilities.
Ratio of magnitudes of Stimulated to Spontaneous emission rates
From equations (2) and (3) we have
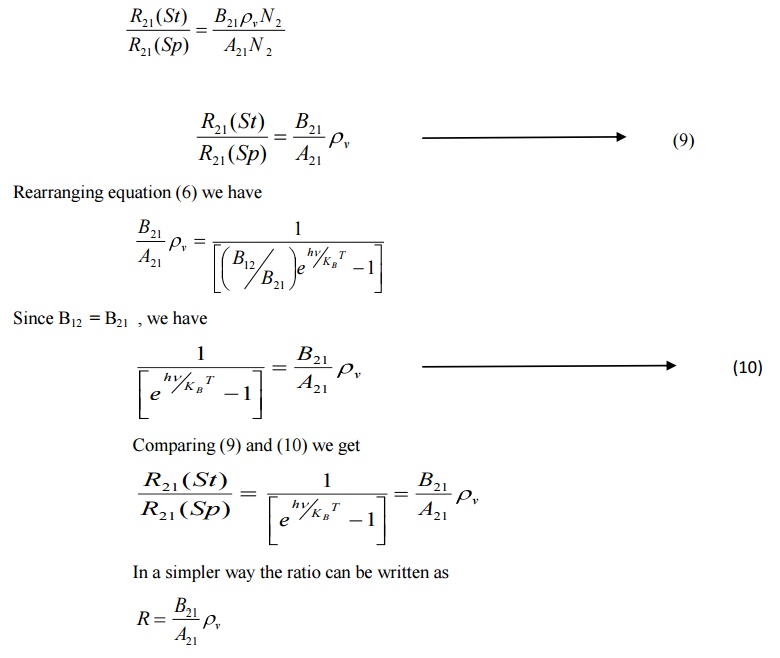
Generally Spontaneous emission is more predominant in the optical region (Ordinary light). To increase the number of coherent photons stimulated emission should dominate over spontaneous emission. To achieve this, an artificial condition called Population Inversion is necessary.
Differences between Stimulated and spontaneous emission of radiation
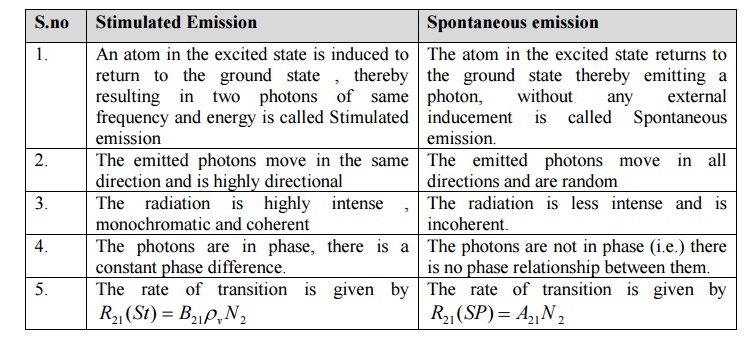
Stimulated Emission
1. An atom in the excited state is induced to return to the ground state , thereby resulting in two photons of same frequency and energy is called Stimulated emission
2. The emitted photons move in the same direction and is highly directional
3. The radiation is highly intense , monochromatic and coherent
4. The photons are in phase, there is a constant phase difference.
Spontaneous emission
1. The atom in the excited state returns to the ground state thereby emitting a photon, without any external inducement is called Spontaneous emission.
2. The emitted photons move in all directions and are random
3. The radiation is less intense and is incoherent.
4. The photons are not in phase (i.e.) there is no phase relationship between them.
Population Inversion:
Population Inversion creates a situation in which the number of atoms in higher energy state is more than that in the lower energy state.
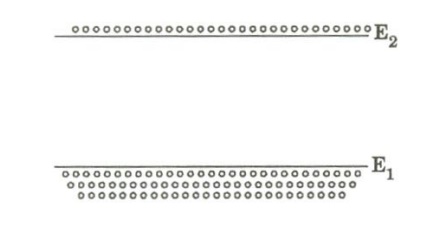
Usually at thermal equilibrium, the number of atoms N2 i.e., the population of atoms at higher energy state is much lesser than the population of the atoms at lower energy state N1 that is N1 > N2 .
The Phenomenon of making N2 > N1 is known as Population Inversion.
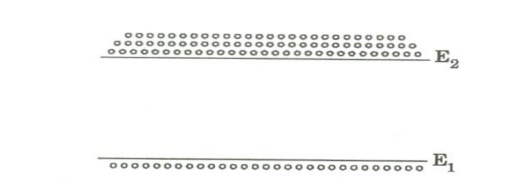
Conditions of Population inversion.
1. There must be at least two energy levels E2 > E1.
2. There must be a source to supply the energy to the medium.
3. The atoms must be continuously raised to the excited state.
Meta stable States
An atom can be excited to a higher level by supplying energy to it. Normally, excited atoms have short life times and release their energy in a matter of nano seconds (10-9) through spontaneous emission. It means atoms do not stay long to be stimulated. As a result, they undergo spontaneous emission and rapidly return to the ground level; thereby population inversion could not be established. In order to do so, the excited atoms are required to ‘wait’ at the upper energy level till a large number of atoms accumulate at that level. In other words, it is necessary that excited state have a longer lifetime. A Meta stable state is such a state. Metastable can be readily obtained in a crystal system containing impurity atoms. These levels lie in the forbidden gap of the host crystal. There could be no population inversion and hence no laser action, if metastable states don’t exist.
Related Topics