Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Precipitation Gravimetry: Quantitative Applications
Quantitative Applications
Although not in common use, precipitation gravimetry still provides a reliable
means for assessing the accuracy of other methods
of analysis or for verifying the composition of standard
reference materials. In this section
we review the general
application of precipitation gravimetry to the analysis of inorganic and organic
compounds.
Inorganic Analysis
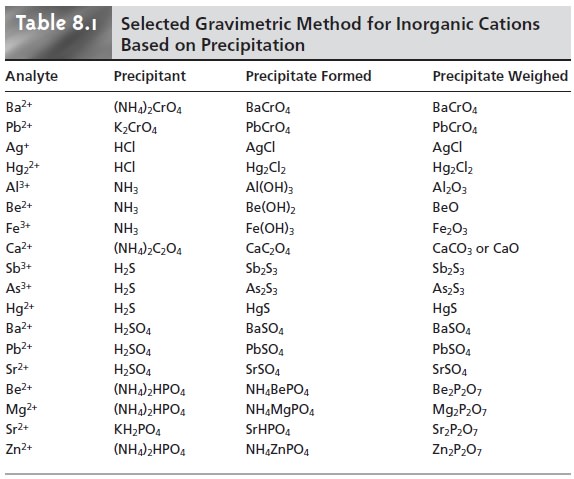
The most important precipitants for inorganic cations are chromate, the halides, hydroxide, oxalate, sulfate, sulfide, and phosphate. A sum- mary of selected methods, grouped by precipitant, is shown in Table 8.1. Many in- organic anions can be determined using the same reactions by reversing the analyte and precipitant.
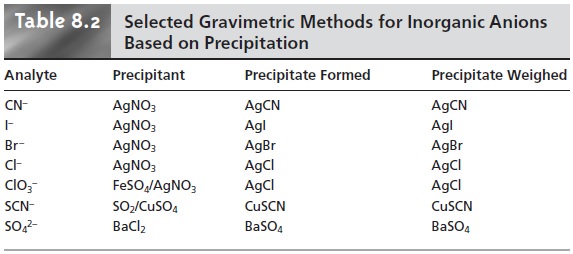
For
example, chromate can
be determined by adding BaCl2 and precipitating BaCrO4. Methods
for other selected
inorganic anions are summarized
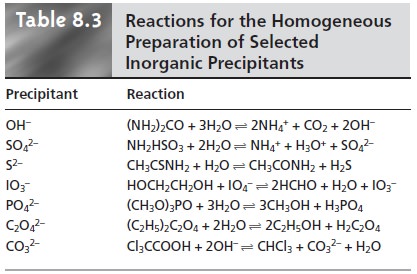
in Table 8.2. Methods for the homogeneous generation of precipitants are shown in Table
8.3.
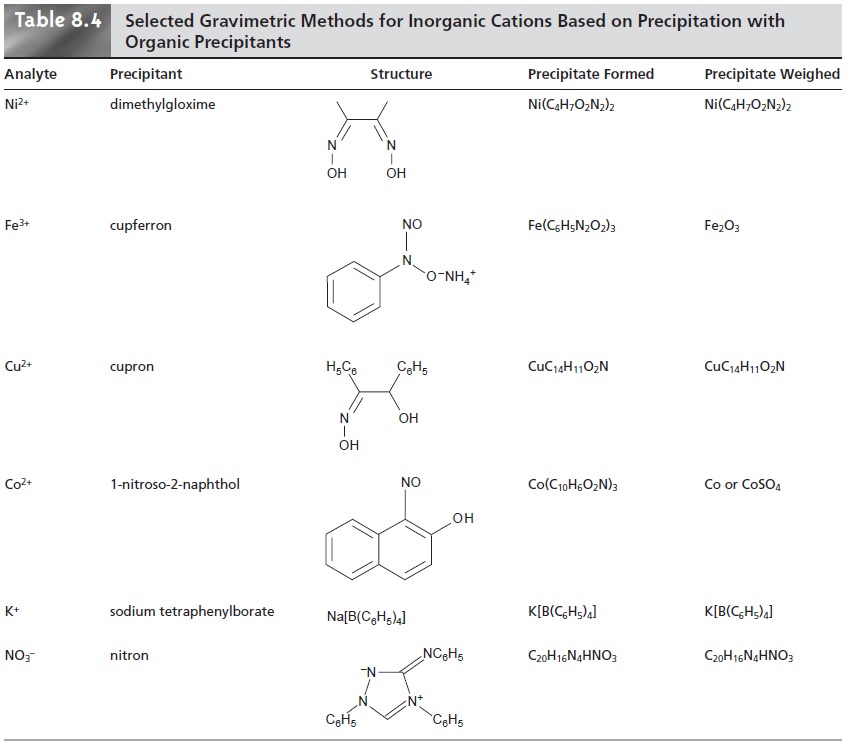
The majority of inorganic precipitants show poor selectivity. Most organic pre- cipitants, however, are selective
for one or two inorganic
ions. Several common or-
ganic precipitants are listed in Table 8.4.
Precipitation gravimetry continues to be listed as a standard method for the analysis of Mg2+ and SO42– in water and wastewater analysis. A description of the procedure for Mg2+ was discussed earlier in Method 8.1. Sulfate is analyzed by pre- cipitating BaSO4, using BaCl2 as the precipitant.
Precipitation is carried out in an acidic solution (acidified to pH 4.5–5.0
with HCl) to prevent the possible precipita- tion of BaCO3 or Ba3(PO4)2 and performed near the solution’s boiling point. The precipitate is digested at 80–90 °C for at least 2 h. Ashless
filter paper pulp is added to
the precipitate to aid in filtration. After
filtering, the precipitate is ignited to con-
stant weight at 800 °C. Alternatively, the precipitate can be filtered
through a fine- porosity fritted glass crucible
(without adding filter
paper pulp) and dried to con-
stant weight at 105 °C.
This procedure is subject to a variety
of errors, including occlusions of Ba(NO3)2, BaCl2, and alkali sulfates.
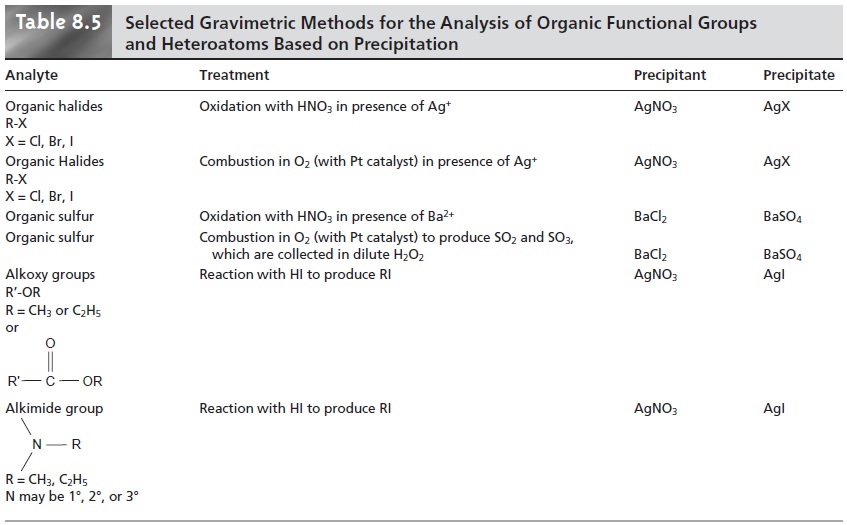
Organic Analysis
Several organic
functional groups or heteroatoms can be deter- mined using gravimetric precipitation methods; examples are
outlined in Table
8.5. Note that the procedures for the alkoxy
and alkimide functional groups are exam- ples of indirect analyses.
Quantitative Calculations
In precipitation gravimetry the relationship between the analyte and the precipitate is determined by the stoichiometry of the relevant reactions. As discussed, gravimetric calculations can be simplified
by
applying the principle of conservation of mass. The following example
demonstrates the application of this approach
to the direct analysis of a single
analyte.

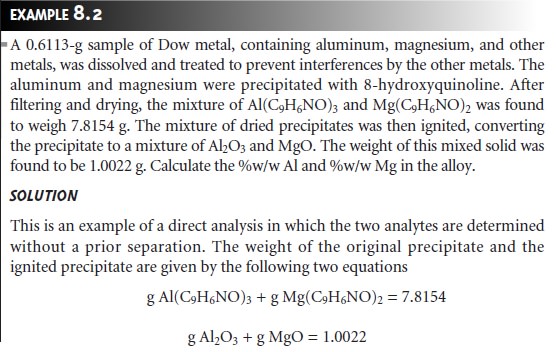
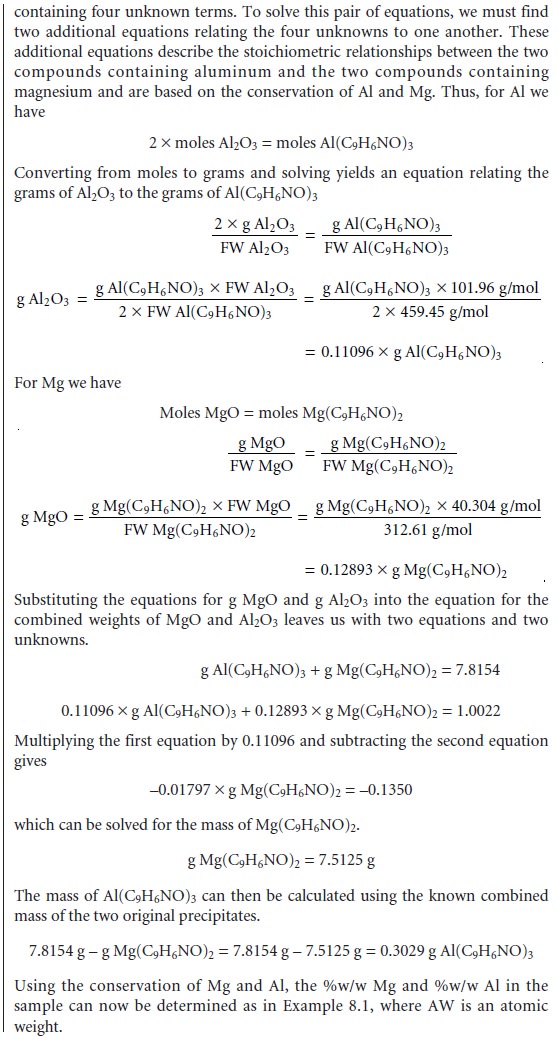
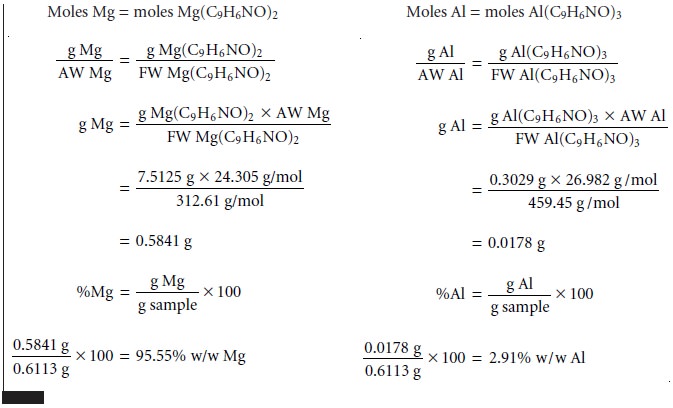
As
discussed earlier, the simultaneous analysis
of samples containing two analytes
requires the isolation of two precipitates. As shown in Example 8.2, conservation of mass can be used to write separate
stoichiometric equations for each precipitate.
These equations can
then be solved
simultaneously for both
analytes.
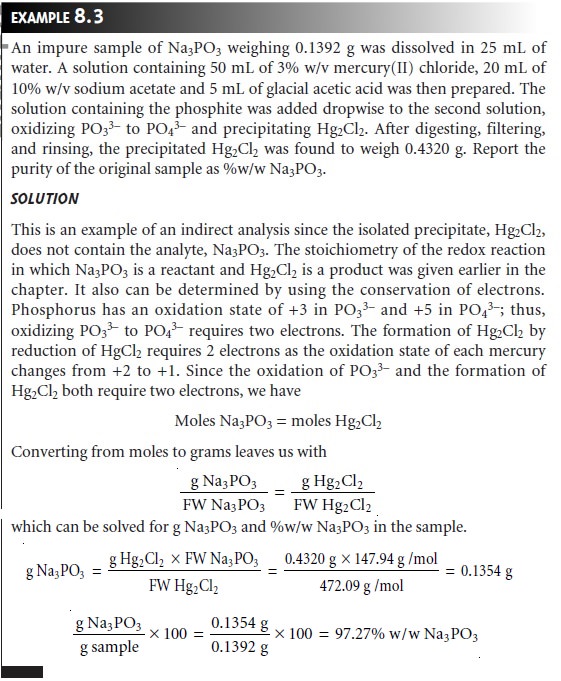
In
an indirect analysis the precipitate does
not contain the
analyte, but is the
product of a reaction involving the analyte. Despite
the additional complexity, a stoichiometric relationship between
the analyte and the precipitate can be written by applying the conservation principles discussed.
Related Topics